Related Research Articles

Central pontine myelinolysis is a neurological condition involving severe damage to the myelin sheath of nerve cells in the pons. It is predominately iatrogenic (treatment-induced), and is characterized by acute paralysis, dysphagia, dysarthria, and other neurological symptoms.

Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the formula C5H4N4O3. It forms ions and salts known as urates and acid urates, such as ammonium acid urate. Uric acid is a product of the metabolic breakdown of purine nucleotides, and it is a normal component of urine. High blood concentrations of uric acid can lead to gout and are associated with other medical conditions, including diabetes and the formation of ammonium acid urate kidney stones.

Anorexia is a medical term for a loss of appetite. While the term outside of the scientific literature is often used interchangeably with anorexia nervosa, many possible causes exist for a loss of appetite, some of which may be harmless, while others indicate a serious clinical condition or pose a significant risk.

Starvation is a severe deficiency in caloric energy intake, below the level needed to maintain an organism's life. It is the most extreme form of malnutrition. In humans, prolonged starvation can cause permanent organ damage and eventually, death. The term inanition refers to the symptoms and effects of starvation. Starvation by outside forces is a crime according to international criminal law and may also be used as a means of torture or execution.

Parenteral nutrition (PN) is the feeding of nutritional products to a person intravenously, bypassing the usual process of eating and digestion. The products are made by pharmaceutical compounding entities or standard pharmaceutical companies. The person receives a nutritional mix according to a formula including glucose, salts, amino acids, lipids and vitamins and dietary minerals. It is called total parenteral nutrition (TPN) or total nutrient admixture (TNA) when no significant nutrition is obtained by other routes, and partial parenteral nutrition (PPN) when nutrition is also partially enteric. It is called peripheral parenteral nutrition (PPN) when administered through vein access in a limb rather than through a central vein as central venous nutrition (CVN).

Hypocalcemia is a medical condition characterized by low calcium levels in the blood serum. The normal range of blood calcium is typically between 2.1–2.6 mmol/L while levels less than 2.1 mmol/L are defined as hypocalcemic. Mildly low levels that develop slowly often have no symptoms. Otherwise symptoms may include numbness, muscle spasms, seizures, confusion, or cardiac arrest.

Ketoacidosis is a metabolic state caused by uncontrolled production of ketone bodies that cause a metabolic acidosis. While ketosis refers to any elevation of blood ketones, ketoacidosis is a specific pathologic condition that results in changes in blood pH and requires medical attention. The most common cause of ketoacidosis is diabetic ketoacidosis but can also be caused by alcohol, medications, toxins, and rarely, starvation.
Tumor lysis syndrome (TLS) is a group of metabolic abnormalities that can occur as a complication from the treatment of cancer, where large amounts of tumor cells are killed off (lysed) from the treatment, releasing their contents into the bloodstream. This occurs most commonly after the treatment of lymphomas and leukemias and in particular when treating non-Hodgkin lymphoma, acute myeloid leukemia, and acute lymphoblastic leukemia. This is a potentially fatal complication and patients at increased risk for TLS should be closely monitored while receiving chemotherapy and should receive preventive measures and treatments as necessary. TLS can also occur on its own although this is less common.
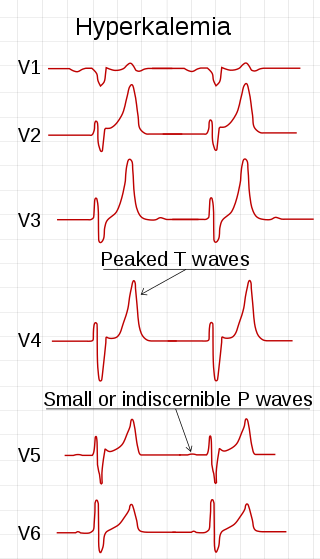
Hyperkalemia is an elevated level of potassium (K+) in the blood. Normal potassium levels are between 3.5 and 5.0 mmol/L (3.5 and 5.0 mEq/L) with levels above 5.5 mmol/L defined as hyperkalemia. Typically hyperkalemia does not cause symptoms. Occasionally when severe it can cause palpitations, muscle pain, muscle weakness, or numbness. Hyperkalemia can cause an abnormal heart rhythm which can result in cardiac arrest and death.

Alcoholic ketoacidosis (AKA) is a specific group of symptoms and metabolic state related to alcohol use. Symptoms often include abdominal pain, vomiting, agitation, a fast respiratory rate, and a specific "fruity" smell. Consciousness is generally normal. Complications may include sudden death.

Electrolyte imbalance, or water-electrolyte imbalance, is an abnormality in the concentration of electrolytes in the body. Electrolytes play a vital role in maintaining homeostasis in the body. They help to regulate heart and neurological function, fluid balance, oxygen delivery, acid–base balance and much more. Electrolyte imbalances can develop by consuming too little or too much electrolyte as well as excreting too little or too much electrolyte. Examples of electrolytes include calcium, chloride, magnesium, phosphate, potassium, and sodium.
Hypermagnesemia is an electrolyte disorder in which there is a high level of magnesium in the blood. Symptoms include weakness, confusion, decreased breathing rate, and decreased reflexes. Complications may include low blood pressure and cardiac arrest.
Hyperchloremia is an electrolyte disturbance in which there is an elevated level of chloride ions in the blood. The normal serum range for chloride is 96 to 106 mEq/L, therefore chloride levels at or above 110 mEq/L usually indicate kidney dysfunction as it is a regulator of chloride concentration. As of now there are no specific symptoms of hyperchloremia; however, it can be influenced by multiple abnormalities that cause a loss of electrolyte-free fluid, loss of hypotonic fluid, or increased administration of sodium chloride. These abnormalities are caused by diarrhea, vomiting, increased sodium chloride intake, renal dysfunction, diuretic use, and diabetes. Hyperchloremia should not be mistaken for hyperchloremic metabolic acidosis as hyperchloremic metabolic acidosis is characterized by two major changes: a decrease in blood pH and bicarbonate levels, as well as an increase in blood chloride levels. Instead those with hyperchloremic metabolic acidosis are usually predisposed to hyperchloremia.

Hypophosphatemia is an electrolyte disorder in which there is a low level of phosphate in the blood. Symptoms may include weakness, trouble breathing, and loss of appetite. Complications may include seizures, coma, rhabdomyolysis, or softening of the bones.

Failure to thrive (FTT), also known as weight faltering or faltering growth, indicates insufficient weight gain or absence of appropriate physical growth in children. FTT is usually defined in terms of weight, and can be evaluated either by a low weight for the child's age, or by a low rate of increase in the weight.
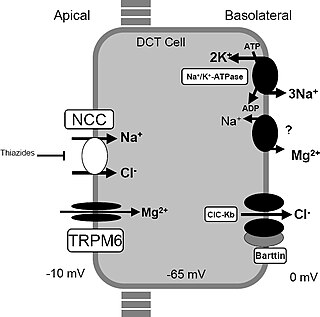
Gitelman syndrome (GS) is an autosomal recessive kidney tubule disorder characterized by low blood levels of potassium and magnesium, decreased excretion of calcium in the urine, and elevated blood pH. It is the most frequent hereditary salt-losing tubulopathy. Gitelman syndrome is caused by disease-causing variants on both alleles of the SLC12A3 gene. The SLC12A3 gene encodes the thiazide-sensitive sodium-chloride cotransporter, which can be found in the distal convoluted tubule of the kidney.
The anion gap is a value calculated from the results of multiple individual medical lab tests. It may be reported with the results of an electrolyte panel, which is often performed as part of a comprehensive metabolic panel.
Magnesium deficiency is an electrolyte disturbance in which there is a low level of magnesium in the body. It can result in multiple symptoms. Symptoms include tremor, poor coordination, muscle spasms, loss of appetite, personality changes, and nystagmus. Complications may include seizures or cardiac arrest such as from torsade de pointes. Those with low magnesium often have low potassium.
Molybdenum deficiency refers to the clinical consequences of inadequate intake of molybdenum in the diet.
Megavitamin-B6 syndrome is a collection of symptoms that can result from chronic supplementation, or acute overdose, of vitamin B6. While it is also known as hypervitaminosis B6, vitamin B6 toxicity and vitamin B6 excess, megavitamin-b6 syndrome is the name used in the ICD-10.
References
- 1 2 "Evidence — Nutrition support for adults: oral nutrition support, enteral tube feeding and parenteral nutrition — Guidance". National Institute for Health and Care Excellence (NICE). 22 February 2006 [Updated 4 August 2017]. Web page with link to full guideline CG32.
- 1 2 Mehanna HM, Moledina J, Travis J (June 2008). "Refeeding syndrome: what it is, and how to prevent and treat it". BMJ. 336 (7659): 1495–8. doi:10.1136/bmj.a301. PMC 2440847 . PMID 18583681.
- 1 2 Doig, GS; Simpson, F; Heighes; Bellomo, R; Chesher, D; Caterson, ID; Reade, MC; Harrigan, PWJ (2015-12-01). "Restricted versus continued standard caloric intake during the management of refeeding syndrome in critically ill adults: a randomised, parallel-group, multicentre, single-blind controlled trial". The Lancet Respiratory Medicine. 3 (12): 943–952. doi:10.1016/S2213-2600(15)00418-X. ISSN 2213-2619. PMID 26597128.
- ↑ Webb GJ, Smith K, Thursby-Pelham F, Smith T, Stroud MA, Da Silva AN (2011). "Complications of emergency refeeding in anorexia nervosa: case series and review". Acute Medicine. 10 (2): 69–76. doi: 10.52964/AMJA.0470 . PMID 22041604.
- ↑ Hippocrates of Kos. De Carnibus. 5th century BCE.
- ↑ The Wars of the Jews by Flavius Josephus. October 2001. p. book V, chapter XIII, paragraph 4. Retrieved 2018-05-22– via www.gutenberg.org.
- ↑ "Researchers play detective to track earliest case of medical malady | The Asahi Shimbun: Breaking News, Japan News and Analysis". The Asahi Shimbun. Retrieved 2024-01-31.
- ↑ Juliana Machado; et al. (March 5, 2009). "Refeeding syndrome, an undiagnosed and forgotten potentially fatal condition". BMJ Case Reports. 2009: bcr07.2008.0521. doi:10.1136/bcr.07.2008.0521. PMC 3028379 . PMID 21686764.
- ↑ Schnitker MA, Mattman PE, Bliss TL (1951). "A clinical study of malnutrition in Japanese prisoners of war". Annals of Internal Medicine. 35 (1): 69–96. doi:10.7326/0003-4819-35-1-69. PMID 14847450.
- ↑ Persinger, Robert B. "Remembering Ebensee 1945 Robert B. Persinger, May 6th 2005". Memorial Ebensee. Archived from the original on 7 October 2011.
- ↑ Nawyn, Kathleen J. "The Liberation of the Ebensee Concentration Camp, May 1945". history.army.mil. U.S. Army Center of Military History. Retrieved 1 October 2018.
- ↑ Burger, GCE; BSandstead, HR; Drummond, J (1945). "Starvation in Western Holland: 1945". The Lancet. 246 (6366): 282–83. doi:10.1016/s0140-6736(45)90738-0.