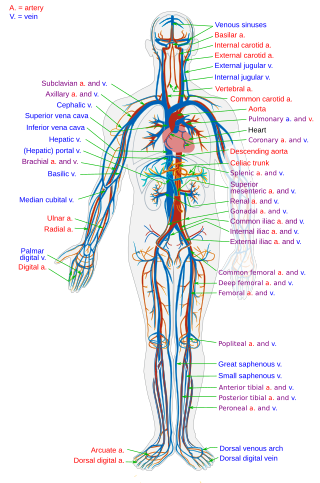
The blood circulatory system is a system of organs that includes the heart, blood vessels, and blood which is circulated throughout the entire body of a human or other vertebrate. It includes the cardiovascular system, or vascular system, that consists of the heart and blood vessels. The circulatory system has two divisions, a systemic circulation or circuit, and a pulmonary circulation or circuit. Some sources use the terms cardiovascular system and vascular system interchangeably with the circulatory system.

In cardiac physiology, cardiac output (CO), also known as heart output and often denoted by the symbols , , or , is the volumetric flow rate of the heart's pumping output: that is, the volume of blood being pumped by a single ventricle of the heart, per unit time. Cardiac output (CO) is the product of the heart rate (HR), i.e. the number of heartbeats per minute (bpm), and the stroke volume (SV), which is the volume of blood pumped from the left ventricle per beat; thus giving the formula:
An air embolism, also known as a gas embolism, is a blood vessel blockage caused by one or more bubbles of air or other gas in the circulatory system. Air can be introduced into the circulation during surgical procedures, lung over-expansion injury, decompression, and a few other causes. In flora, air embolisms may also occur in the xylem of vascular plants, especially when suffering from water stress.

dextro-Transposition of the great arteries is a potentially life-threatening birth defect in the large arteries of the heart. The primary arteries are transposed.

Mitral stenosis is a valvular heart disease characterized by the narrowing of the opening of the mitral valve of the heart. It is almost always caused by rheumatic valvular heart disease. Normally, the mitral valve is about 5 cm2 during diastole. Any decrease in area below 2 cm2 causes mitral stenosis. Early diagnosis of mitral stenosis in pregnancy is very important as the heart cannot tolerate increased cardiac output demand as in the case of exercise and pregnancy. Atrial fibrillation is a common complication of resulting left atrial enlargement, which can lead to systemic thromboembolic complications such as stroke.

Atrial septal defect (ASD) is a congenital heart defect in which blood flows between the atria of the heart. Some flow is a normal condition both pre-birth and immediately post-birth via the foramen ovale; however, when this does not naturally close after birth it is referred to as a patent (open) foramen ovale (PFO). It is common in patients with a congenital atrial septal aneurysm (ASA).

The jugular venous pressure is the indirectly observed pressure over the venous system via visualization of the internal jugular vein. It can be useful in the differentiation of different forms of heart and lung disease. Classically three upward deflections and two downward deflections have been described.
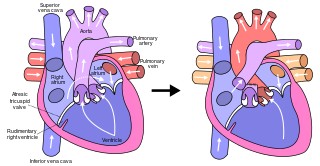
The Fontan procedure or Fontan–Kreutzer procedure is a palliative surgical procedure used in children with univentricular hearts. It involves diverting the venous blood from the inferior vena cava (IVC) and superior vena cava (SVC) to the pulmonary arteries. The procedure varies for differing congenital heart pathologies. For example in tricuspid atresia, the procedure can be done where the blood does not pass through the morphologic right ventricle; i.e., the systemic and pulmonary circulations are placed in series with the functional single ventricle. Whereas in hypoplastic left heart syndrome, the heart is more reliant on the more functional right ventricle to provide blood flow to the systemic circulation. The procedure was initially performed in 1968 by Francis Fontan and Eugene Baudet from Bordeaux, France, published in 1971, simultaneously described in 1971 by Guillermo Kreutzer from Buenos Aires, Argentina, and finally published in 1973.

Cardiac catheterization is the insertion of a catheter into a chamber or vessel of the heart. This is done both for diagnostic and interventional purposes.

Cardiogenic shock (CS) is a medical emergency resulting from inadequate blood flow due to the dysfunction of the ventricles of the heart. Signs of inadequate blood flow include low urine production, cool arms and legs, and decreased level of consciousness. People may also have a severely low blood pressure and heart rate.

The atrium is one of the two upper chambers in the heart that receives blood from the circulatory system. The blood in the atria is pumped into the heart ventricles through the atrioventricular mitral and tricuspid heart valves.

In cardiac physiology, preload is the amount of sarcomere stretch experienced by cardiac muscle cells, called cardiomyocytes, at the end of ventricular filling during diastole. Preload is directly related to ventricular filling. As the relaxed ventricle fills during diastole, the walls are stretched and the length of sarcomeres increases. Sarcomere length can be approximated by the volume of the ventricle because each shape has a conserved surface-area-to-volume ratio. This is useful clinically because measuring the sarcomere length is destructive to heart tissue. It requires cutting out a piece of cardiac muscle to look at the sarcomeres under a microscope. It is currently not possible to directly measure preload in the beating heart of a living animal. Preload is estimated from end-diastolic ventricular pressure and is measured in millimeters of mercury (mmHg).
Pulsus paradoxus, also paradoxic pulse or paradoxical pulse, is an abnormally large decrease in stroke volume, systolic blood pressure and pulse wave amplitude during inspiration. Pulsus paradoxus is not related to pulse rate or heart rate, and it is not a paradoxical rise in systolic pressure. Normally, blood pressure drops less precipitously than 10 mmHg during inhalation. Pulsus paradoxus is a sign that is indicative of several conditions most commonly pericardial effusion.
Central venous pressure (CVP) is the blood pressure in the venae cavae, near the right atrium of the heart. CVP reflects the amount of blood returning to the heart and the ability of the heart to pump the blood back into the arterial system. CVP is often a good approximation of right atrial pressure (RAP), although the two terms are not identical, as a pressure differential can sometimes exist between the venae cavae and the right atrium. CVP and RAP can differ when arterial tone is altered. This can be graphically depicted as changes in the slope of the venous return plotted against right atrial pressure.
Impedance cardiography (ICG) is a non-invasive technology measuring total electrical conductivity of the thorax and its changes in time to process continuously a number of cardiodynamic parameters, such as stroke volume (SV), heart rate (HR), cardiac output (CO), ventricular ejection time (VET), pre-ejection period and used to detect the impedance changes caused by a high-frequency, low magnitude current flowing through the thorax between additional two pairs of electrodes located outside of the measured segment. The sensing electrodes also detect the ECG signal, which is used as a timing clock of the system.

Right ventricular hypertrophy (RVH) is a condition defined by an abnormal enlargement of the cardiac muscle surrounding the right ventricle. The right ventricle is one of the four chambers of the heart. It is located towards the lower-end of the heart and it receives blood from the right atrium and pumps blood into the lungs.
A cardiac shunt is a pattern of blood flow in the heart that deviates from the normal circuit of the circulatory system. It may be described as right-left, left-right or bidirectional, or as systemic-to-pulmonary or pulmonary-to-systemic. The direction may be controlled by left and/or right heart pressure, a biological or artificial heart valve or both. The presence of a shunt may also affect left and/or right heart pressure either beneficially or detrimentally.
The following outline is provided as an overview of and topical guide to cardiology, the branch of medicine dealing with disorders of the human heart. The field includes medical diagnosis and treatment of congenital heart defects, coronary artery disease, heart failure, valvular heart disease and electrophysiology. Physicians who specialize in cardiology are called cardiologists.

Heart failure with preserved ejection fraction (HFpEF) is a form of heart failure in which the ejection fraction – the percentage of the volume of blood ejected from the left ventricle with each heartbeat divided by the volume of blood when the left ventricle is maximally filled – is normal, defined as greater than 50%; this may be measured by echocardiography or cardiac catheterization. Approximately half of people with heart failure have preserved ejection fraction, while the other half have a reduction in ejection fraction, called heart failure with reduced ejection fraction (HFrEF).
Raghib syndrome is rare a congenital heart defect where the left superior vena cava (LSVC) is draining into the left atrium in addition to an absent coronary sinus and an atrial septal defect. This can be considered a dangerous heart condition because it puts the individual at a high risk of stroke. Other defects that are often associated with Raghib syndrome can include ventricular septal defects, enlargement of the tricuspid annulus, and pulmonary stenosis. While this is considered an extremely rare developmental complex, cases regarding a persistent left superior vena cava (PLSVC) are relatively common among congenital heart defects. It is also important to note that the PLSVC often drains into the right atrium, and only drains into the left atrium in approximately 10 to 20% of individuals with the defect.














