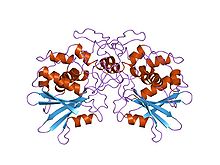
Ricin ( RY-sin) is a lectin (a carbohydrate-binding protein) and a highly potent toxin produced in the seeds of the castor oil plant, Ricinus communis. The median lethal dose (LD50) of ricin for mice is around 22 micrograms per kilogram of body weight via intraperitoneal injection. Oral exposure to ricin is far less toxic. An estimated lethal oral dose in humans is approximately one milligram per kilogram of body weight.

Shiga toxins are a family of related toxins with two major groups, Stx1 and Stx2, expressed by genes considered to be part of the genome of lambdoid prophages. The toxins are named after Kiyoshi Shiga, who first described the bacterial origin of dysentery caused by Shigella dysenteriae. Shiga-like toxin (SLT) is a historical term for similar or identical toxins produced by Escherichia coli. The most common sources for Shiga toxin are the bacteria S. dysenteriae and some serotypes of Escherichia coli (STEC), which includes serotypes O157:H7, and O104:H4.
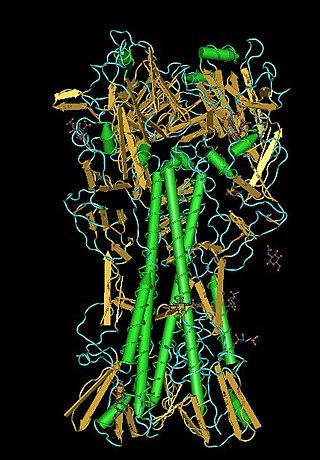
Lectins are carbohydrate-binding proteins that are highly specific for sugar groups that are part of other molecules, so cause agglutination of particular cells or precipitation of glycoconjugates and polysaccharides. Lectins have a role in recognition at the cellular and molecular level and play numerous roles in biological recognition phenomena involving cells, carbohydrates, and proteins. Lectins also mediate attachment and binding of bacteria, viruses, and fungi to their intended targets.

Ribonuclease is a type of nuclease that catalyzes the degradation of RNA into smaller components. Ribonucleases can be divided into endoribonucleases and exoribonucleases, and comprise several sub-classes within the EC 2.7 and 3.1 classes of enzymes.

An exotoxin is a toxin secreted by bacteria. An exotoxin can cause damage to the host by destroying cells or disrupting normal cellular metabolism. They are highly potent and can cause major damage to the host. Exotoxins may be secreted, or, similar to endotoxins, may be released during lysis of the cell. Gram negative pathogens may secrete outer membrane vesicles containing lipopolysaccharide endotoxin and some virulence proteins in the bounding membrane along with some other toxins as intra-vesicular contents, thus adding a previously unforeseen dimension to the well-known eukaryote process of membrane vesicle trafficking, which is quite active at the host–pathogen interface.

Abrin is an extremely toxic toxalbumin found in the seeds of the rosary pea, Abrus precatorius. It has a median lethal dose of 0.7 micrograms per kilogram of body mass when given to mice intravenously. The median toxic dose for humans ranges from 10 to 1000 micrograms per kilogram when ingested and is 3.3 micrograms per kilogram when inhaled.

Gelonin is a type 1 ribosome-inactivating protein and toxin of approximately 30 kDa found in the seeds of the Himalayan plant Gelonium multiflorum. In cell-free systems gelonin exerts powerful N-glycosidase activity on the 28S rRNA unit of eukaryotic ribosomes by cleaving out adenine at the 4324 site. Gelonin lacks carbohydrate-binding domains so it is unable to cross the plasma membrane, making it highly effective only in cell free systems.
Saporin is a protein that is useful in biological research applications, especially studies of behavior. Saporins are so-called ribosome inactivating proteins (RIPs), due to its N-glycosidase activity, from the seeds of Saponaria officinalis. It was first described by Fiorenzo Stirpe and his colleagues in 1983 in an article that illustrated the unusual stability of the protein.

EF-G is a prokaryotic elongation factor involved in mRNA translation. As a GTPase, EF-G catalyzes the movement (translocation) of transfer RNA (tRNA) and messenger RNA (mRNA) through the ribosome.
Beetin is a ribosome-inactivating protein found in the leaves of sugar beets, Beta vulgaris L, specifically attacking plant ribosomes. Sugar beet, beetins, that have been isolated meet all the criteria to be classified as single chain ribosome inactivating proteins that are highly toxic to mammalian ribosomes but non-toxic to intact cultured mammalian cells. Beetin expression occurs when there is a viral infection of the plant. The different levels of glycosylation of the same polypeptide chain result in the two forms of beetin. Beetin exhibits these two primary forms with apparent Mr values of 27 000 (BE27) and 29 000 (BE29) along with possessing glycan chains. Beetins are a type-I (single-chain) proteins with N-glycoside activity. Since it has been discovered that beetin is mostly concentrated in the intercellular fluid, its presence in the remaining parts of the leaf may be below the limit of detection rather than being nonexistent. The expression of beetin is only found in mature plants, but is present in all developing stages.

Toxalbumins are toxic plant proteins that disable ribosomes and thereby inhibit protein synthesis, producing severe cytotoxic effects in multiple organ systems. They are dimers held together by a disulfide bond and comprise a lectin part which binds to the cell membrane and enables the toxin part to gain access to the cell contents. Toxalbumins are similar in structure to AB toxins found in cholera, tetanus, diphtheria, botulinum and others; and their physiological and toxic properties are similar to those of viperine snake venom.

Antiviral proteins are proteins that are induced by human or animal cells to interfere with viral replication. These proteins are isolated to inhibit the virus from replicating in a host's cells and stop it from spreading to other cells. The Pokeweed antiviral protein and the Zinc-Finger antiviral protein are two major antiviral proteins that have undergone several tests for viruses, including HIV and influenza.

In molecular biology, the CRM domain is an approximately 100-amino acid RNA-binding domain. The name CRM has been suggested to reflect the functions established for four characterised members of the family: Zea mays (Maize) CRS1, CAF1 and CAF2 proteins and the Escherichia coli protein YhbY. Proteins containing the CRM domain are found in eubacteria, archaea, and plants. The CRM domain is represented as a stand-alone protein in archaea and bacteria, and in single- and multi-domain proteins in plants. It has been suggested that prokaryotic CRM proteins existed as ribosome-associated proteins prior to the divergence of archaea and bacteria, and that they were co-opted in the plant lineage as RNA binding modules by incorporation into diverse protein contexts. Plant CRM domains are predicted to reside not only in the chloroplast, but also in the mitochondrion and the nucleo/cytoplasmic compartment. The diversity of the CRM domain family in plants suggests a diverse set of RNA targets.
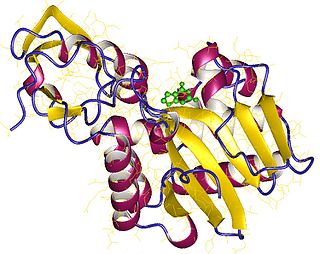
rRNA N-glycosylase is an enzyme with systematic name rRNA N-glycohydrolase. This enzyme catalyses the following chemical reaction

Lactase-like is a protein that in humans is encoded by the LCTL gene. Lactase-like is a glycosidase enzyme.
Volkensin is a eukaryotic ribosome-inactivating protein found in the Adenia volkensii plant. It is a glycoprotein with two subunits A and B. A subunit is linked to B subunit with disulfide bridges and non-covalent bonds. B subunit is responsible for binding to the galactosyl-terminated receptors on the cell membrane that allows the entry the A subunit of the toxin into the cell, which performs the inhibitory function. Volkensin is a galactose specific lectin that can inhibit protein synthesis in whole cells and in cell-free lysates. This protein can be included into the category of risin like toxins and it resembles modeccin, the toxin of Adenia digitata. Although very similar in composition, volkensin contains more cysteine residues and more than twice as much sugar than modeccin, due to high content of galactose and mannose. In addition, volkensin is able to inhibit protein synthesis at concentrations 10 times lower than required for modeccin. From gene sequencing analysis, volkensin was found to be coded by 1569-bp ORF, that is 523 amino acid residues without introns. The internal linker sequence is 45 bp. The active site of the A subunit contains Ser203, a novel residue that is conserved in all ribosome inactivating proteins.
Spiroplasma poulsonii are bacteria of the genus Spiroplasma that are commonly endosymbionts of flies. These bacteria live in the hemolymph of the flies, where they can act as reproductive manipulators or defensive symbionts.
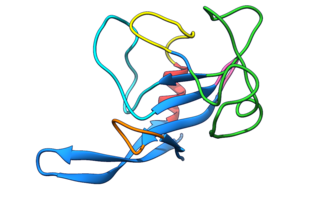
Fungal ribotoxins are a group of extracellular ribonucleases (RNases) secreted by fungi. Their most notable characteristic is their extraordinary specificity. They inactivate ribosomes by cutting a single phosphodiester bond of the rRNA that is found in a universally conserved sequence. This cleavage leads to cell death by apoptosis. However, since they are extracellular proteins, they must first enter the cells that constitute their target to exert their cytotoxic action. This entry constitutes the rate-determining step of their action.
Modeccin is a toxic lectin, a group of glycoproteins capable of binding specifically to sugar moieties. Different toxic lectins are present in seeds of different origin. Modeccin is found in the roots of the African plant Adenia digitata. These roots are often mistaken for edible roots, which has led to some cases of intoxication. Sometimes the fruit is eaten, or a root extract is drunk as a manner of suicide.
Robin is a lectin, a carbohydrate-binding protein, and a highly potent toxin. Robin is a type of toxalbumin.