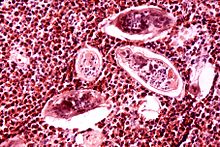
Schistosomiasis, also known as snail fever, bilharzia, and Katayama fever, is a disease caused by parasitic flatworms called schistosomes. The urinary tract or the intestines may be infected. Symptoms include abdominal pain, diarrhea, bloody stool, or blood in the urine. Those who have been infected for a long time may experience liver damage, kidney failure, infertility, or bladder cancer. In children, it may cause poor growth and learning difficulty.

Trematoda is a class of flatworms known as flukes or trematodes. They are obligate internal parasites with a complex life cycle requiring at least two hosts. The intermediate host, in which asexual reproduction occurs, is usually a snail. The definitive host, where the flukes sexually reproduce, is a vertebrate. Infection by trematodes can cause disease in all five traditional vertebrate classes: mammals, birds, amphibians, reptiles, and fish.
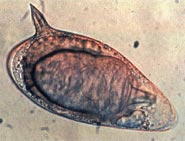
Schistosoma is a genus of trematodes, commonly known as blood flukes. They are parasitic flatworms responsible for a highly significant group of infections in humans termed schistosomiasis, which is considered by the World Health Organization as the second-most socioeconomically devastating parasitic disease, with hundreds of millions infected worldwide.

Clonorchis sinensis, the Chinese liver fluke, is a liver fluke belonging to the class Trematoda, phylum Platyhelminthes. It infects fish-eating mammals, including humans. In humans, it infects the common bile duct and gall bladder, feeding on bile. It was discovered by British physician James McConnell at the Medical College Hospital in Calcutta (Kolkata) in 1874. The first description was given by Thomas Spencer Cobbold, who named it Distoma sinense. The fluke passes its lifecycle in three different hosts, namely freshwater snail as first intermediate hosts, freshwater fish as second intermediate host, and mammals as definitive hosts.

Trematodes are parasitic flatworms of the class Trematoda, specifically parasitic flukes with two suckers: one ventral and the other oral. Trematodes are covered by a tegument, that protects the organism from the environment by providing secretory and absorptive functions.
Schistosoma japonicum is an important parasite and one of the major infectious agents of schistosomiasis. This parasite has a very wide host range, infecting at least 31 species of wild mammals, including nine carnivores, 16 rodents, one primate (human), two insectivores and three artiodactyls and therefore it can be considered a true zoonosis. Travelers should be well-aware of where this parasite might be a problem and how to prevent the infection. S. japonicum occurs in the Far East, such as China, the Philippines, Indonesia and Southeast Asia.

Trichobilharzia regenti is a neuropathogenic parasitic flatworm of birds which also causes cercarial dermatitis in humans. The species was originally described in 1998 in the Czech Republic and afterwards it was detected also in other European countries, e.g. Denmark, Germany, France, Iceland, Poland, Switzerland, or Russia, and even in Iran. For its unique neurotropic behaviour in vertebrate hosts, the host-parasite interactions are extensively studied in terms of molecular biology, biochemistry and immunology.

Schistosoma mansoni is a water-borne parasite of humans, and belongs to the group of blood flukes (Schistosoma). The adult lives in the blood vessels near the human intestine. It causes intestinal schistosomiasis. Clinical symptoms are caused by the eggs. As the leading cause of schistosomiasis in the world, it is the most prevalent parasite in humans. It is classified as a neglected tropical disease. As of 2021, the World Health Organization reports that 251.4 million people have schistosomiasis and most of it is due to S. mansoni. It is found in Africa, the Middle East, the Caribbean, Brazil, Venezuela and Suriname.

Swimmer's itch, cercarial dermatitis or schistosome dermatitis is a short-term allergic contact dermatitis occurring in the skin of humans that have been infected by water-borne schistosomes, a type of flatworm. It is common in freshwater, brackish and marine habitats worldwide. The incidence of this condition may be increasing, although this may be attributed to better monitoring and reporting. Nevertheless, the condition is considered to be an emerging infectious disease.

Schistosoma intercalatum is a parasitic worm found in parts of western and central Africa. There are two strains: the Lower Guinea strain and the Zaire strain. S. intercalatum is one of the major agents of the rectal form of schistosomiasis, also called bilharzia. It is a trematode, and being part of the genus Schistosoma, it is commonly referred to as a blood-fluke since the adult resides in blood vessels.

Theodor Maximilian Bilharz was a German physician who made pioneering discoveries in the field of parasitology. His contributions led to the foundation of tropical medicine. He is best remembered as the discoverer of the blood fluke Schistosoma haematobium, the causative parasite of bloody urine (haematuria) known since ancient times in Egypt. The parasite, as the cause of bladder cancer, is declared by the International Agency for Research on Cancer as Group 1 carcinogen. The infection is known by an eponymous term bilharzia or bilharziasis, as well as by schistosomiasis.
Schistosoma indicum is a species of digenetic trematode in the family Schistosomatidae. The parasite is widespread in domestic animals in India and other Asian countries.

A Schistosomiasis vaccine is a vaccine against Schistosomiasis, a parasitic disease caused by several species of fluke of the genus Schistosoma. No effective vaccine for the disease exists yet. Schistosomiasis affects over 200 million people worldwide, mainly in rural agricultural and peri-urban areas of the third world, and approximately 10% suffer severe health complications from the infection. While chemotherapeutic drugs, such as praziquantel, oxamniquine and metrifonate both no longer on the market, are currently considered safe and effective for the treatment of schistosomiasis, reinfection occurs frequently following drug treatment, thus a vaccine is sought to provide long-term treatment. Additionally, experimental vaccination efforts have been successful in animal models of schistosomiasis.
Schistosoma mekongi is a species of trematodes, also known as flukes. It is one of the five major schistosomes that account for all human infections, the other four being S. haematobium, S. mansoni, S. japonicum, and S. intercalatum. This trematode causes schistosomiasis in humans.

Schistosoma spindale is a species of digenetic trematode in the family Schistosomatidae. It causes intestinal schistosomiasis in the ruminants.

Bivitellobilharzia nairi is a species of trematodes, part of the family Schistosomatidae. This is a fairly new identified endoparasite that was found in 1945 by Mudaliar and Ramanujachari, who first recorded the parasite in India. Researchers collected fecal samples of the Indian rhinoceros and were startled to find B. nairi eggs.

Metagonimus yokogawai, or the Yokogawa fluke, is a species of a trematode, or fluke worm, in the family Heterophyidae.
Schistosoma bovis is a two-host blood fluke, that causes intestinal schistosomiasis in ruminants in North Africa, Mediterranean Europe and the Middle East. S. bovis is mostly transmitted by Bulinus freshwater snail species. It is one of nine haematobium group species and exists in the same geographical areas as Schistosoma haematobium, with which it can hybridise. S. bovis-haematobium hybrids can infect humans, and have been reported in Senegal since 2009, and a 2013 outbreak in Corsica.
Carcinogenic parasites are parasitic organisms that depend on other organisms for their survival, and cause cancer in such hosts. Three species of flukes (trematodes) are medically-proven carcinogenic parasites, namely the urinary blood fluke, the Southeast Asian liver fluke and the Chinese liver fluke. S. haematobium is prevalent in Africa and the Middle East, and is the leading cause of bladder cancer. O. viverrini and C. sinensis are both found in eastern and southeastern Asia, and are responsible for cholangiocarcinoma. The International Agency for Research on Cancer declared them in 2009 as a Group 1 biological carcinogens in humans.
Schistosoma hippopotami is a species of digenetic trematode that belongs to the genus of blood flukes (Schistosoma) that is found in sub-Saharan Africa. It primarily infects African hippopotamuses and has a more limited host range compared to other Schistosoma species.