Related Research Articles
General anaesthetics are often defined as compounds that induce a loss of consciousness in humans or loss of righting reflex in animals. Clinical definitions are also extended to include an induced coma that causes lack of awareness to painful stimuli, sufficient to facilitate surgical applications in clinical and veterinary practice. General anaesthetics do not act as analgesics and should also not be confused with sedatives. General anaesthetics are a structurally diverse group of compounds whose mechanisms encompasses multiple biological targets involved in the control of neuronal pathways. The precise workings are the subject of some debate and ongoing research.

Nitrous oxide, commonly known as laughing gas, nitrous, or nos, is a chemical compound, an oxide of nitrogen with the formula N
2O. At room temperature, it is a colourless non-flammable gas, and has a slightly sweet scent and taste. At elevated temperatures, nitrous oxide is a powerful oxidiser similar to molecular oxygen.

The respiratory system is a biological system consisting of specific organs and structures used for gas exchange in animals and plants. The anatomy and physiology that make this happen varies greatly, depending on the size of the organism, the environment in which it lives and its evolutionary history. In land animals the respiratory surface is internalized as linings of the lungs. Gas exchange in the lungs occurs in millions of small air sacs; in mammals and reptiles these are called alveoli, and in birds they are known as atria. These microscopic air sacs have a very rich blood supply, thus bringing the air into close contact with the blood. These air sacs communicate with the external environment via a system of airways, or hollow tubes, of which the largest is the trachea, which branches in the middle of the chest into the two main bronchi. These enter the lungs where they branch into progressively narrower secondary and tertiary bronchi that branch into numerous smaller tubes, the bronchioles. In birds the bronchioles are termed parabronchi. It is the bronchioles, or parabronchi that generally open into the microscopic alveoli in mammals and atria in birds. Air has to be pumped from the environment into the alveoli or atria by the process of breathing which involves the muscles of respiration.

Halothane, sold under the brand name Fluothane among others, is a general anaesthetic. It can be used to induce or maintain anaesthesia. One of its benefits is that it does not increase the production of saliva, which can be particularly useful in those who are difficult to intubate. It is given by inhalation.
Diffusing capacity of the lung (DL) measures the transfer of gas from air in the lung, to the red blood cells in lung blood vessels. It is part of a comprehensive series of pulmonary function tests to determine the overall ability of the lung to transport gas into and out of the blood. DL, especially DLCO, is reduced in certain diseases of the lung and heart. DLCO measurement has been standardized according to a position paper by a task force of the European Respiratory and American Thoracic Societies.
Dead space is the volume of air that is inhaled that does not take part in the gas exchange, because it either remains in the conducting airways or reaches alveoli that are not perfused or poorly perfused. It means that not all the air in each breath is available for the exchange of oxygen and carbon dioxide. Mammals breathe in and out of their lungs, wasting that part of the inhalation which remains in the conducting airways where no gas exchange can occur.

Isoflurane, sold under the brand name Forane among others, is a general anesthetic. It can be used to start or maintain anesthesia; however, other medications are often used to start anesthesia rather than isoflurane, due to airway irritation with isoflurane. Isoflurane is given via inhalation.

Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane itself is mainly of theoretical interest but many of its derivatives are of commercial or biological significance.

An anesthetic or anaesthetic is a drug used to induce anesthesia — in other words, to result in a temporary loss of sensation or awareness. They may be divided into two broad classes: general anesthetics, which result in a reversible loss of consciousness, and local anesthetics, which cause a reversible loss of sensation for a limited region of the body without necessarily affecting consciousness.
Awareness under anesthesia, also referred to as intraoperative awareness or accidental awareness during general anesthesia (AAGA), is a rare complication of general anesthesia where patients regain varying levels of consciousness during their surgical procedures. While anesthesia awareness is possible without resulting in any long-term memory, it is also possible for the victim to have awareness with explicit recall, where victims can remember the events related to their surgery.
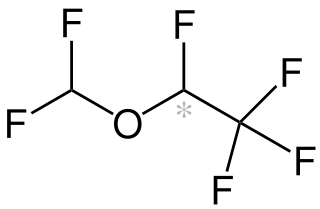
Desflurane is a highly fluorinated methyl ethyl ether used for maintenance of general anesthesia. Like halothane, enflurane, and isoflurane, it is a racemic mixture of (R) and (S) optical isomers (enantiomers). Together with sevoflurane, it is gradually replacing isoflurane for human use, except in economically undeveloped areas, where its high cost precludes its use. It has the most rapid onset and offset of the volatile anesthetic drugs used for general anesthesia due to its low solubility in blood.
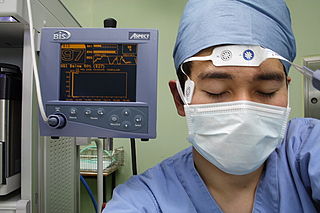
Bispectral index (BIS) is one of several technologies used to monitor depth of anesthesia. BIS monitors are used to supplement Guedel's classification system for determining depth of anesthesia. Titrating anesthetic agents to a specific bispectral index during general anesthesia in adults allows the anesthetist to adjust the amount of anesthetic agent to the needs of the patient, possibly resulting in a more rapid emergence from anesthesia. Use of the BIS monitor could reduce the incidence of intraoperative awareness during anaesthesia. The exact details of the algorithm used to create the BIS index have not been disclosed by the company that developed it.

An inhalational anesthetic is a chemical compound possessing general anesthetic properties that can be delivered via inhalation. They are administered through a face mask, laryngeal mask airway or tracheal tube connected to an anesthetic vaporiser and an anesthetic delivery system. Agents of significant contemporary clinical interest include volatile anesthetic agents such as isoflurane, sevoflurane and desflurane, as well as certain anesthetic gases such as nitrous oxide and xenon.
Minimum alveolar concentration or MAC is the concentration of a vapour in the alveoli of the lungs that is needed to prevent movement in 50% of subjects in response to surgical (pain) stimulus. MAC is used to compare the strengths, or potency, of anaesthetic vapours. The concept of MAC was first introduced in 1965.

Methoxyflurane, sold under the brand name Penthrox among others, is an inhaled medication primarily used to reduce pain following trauma. It may also be used for short episodes of pain as a result of medical procedures. Onset of pain relief is rapid and of a short duration. Use is only recommended with direct medical supervision.
A pulmonary shunt refers to the passage of deoxygenated blood from the right side of the heart to the left without participation in gas exchange in the pulmonary capillaries. It is a pathological condition that results when the alveoli of the lungs are perfused with blood as normal, but ventilation fails to supply the perfused region. In other words, the ventilation/perfusion ratio is zero.
Dental anesthesia is the application of anesthesia to dentistry. It includes local anesthetics, sedation, and general anesthesia.
The Fink effect, also known as "diffusion anoxia", "diffusion hypoxia", or the "second gas effect", is a factor that influences the pO2 (partial pressure of oxygen) within the pulmonary alveoli. When water-soluble gases such as anesthetic agent N2O (nitrous oxide) are breathed in large quantities they can be dissolved in body fluids rapidly. This leads to a temporary increase in both the concentrations and partial pressures of oxygen and carbon dioxide in the alveoli.
In the study of inhaled anesthetics, the concentration effect is the increase in the rate that the Fa /Fi ratio rises as the alveolar concentration of that gas is increased. In simple terms, the higher the concentration of gas administered, the faster the alveolar concentration of that gas approaches the inspired concentration. In modern practice it is only relevant for nitrous oxide since other inhaled anesthetics are delivered at much lower concentrations due to their higher potency.
Blood–gas partition coefficient, also known as Ostwald coefficient for blood–gas, is a term used in pharmacology to describe the solubility of inhaled general anesthetics in blood. According to Henry's law, the ratio of the concentration in blood to the concentration in gas that is in contact with that blood, when the partial pressure in both compartments is equal, is nearly constant at sufficiently low concentrations. The partition coefficient is defined as this ratio and, therefore, has no units. The concentration of the anesthetic in blood includes the portion that is undissolved in plasma and the portion that is dissolved. The more soluble the inhaled anesthetic is in blood compared to in air, the more it binds to plasma proteins in the blood and the higher the blood–gas partition coefficient.
References
- ↑ Korman, B.; Mapleson, W. W. (May 1997). "Concentration and second gas effects: can the accepted explanation be improved?". British Journal of Anaesthesia. 78 (5): 618–625. doi: 10.1093/bja/78.5.618 . PMID 9175984.
- Mapleson, W. W.; Korman, B. (Dec 1998). "Concentration and second-gas effects in the water analogue". British Journal of Anaesthesia. 81 (6): 837–843. doi: 10.1093/bja/81.6.837 . PMID 10211005.
- Errata British Journal of Anaesthesia 1997;79:268