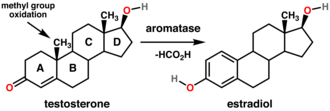
The ovary is an organ found in the female reproductive system that produces an ovum. When released, this travels down the fallopian tube into the uterus, where it may become fertilized by a sperm. There is an ovary found on each side of the body. The ovaries also secrete hormones that play a role in the menstrual cycle and fertility. The ovary progresses through many stages beginning in the prenatal period through menopause. It is also an endocrine gland because of the various hormones that it secretes.

The menstrual cycle is a series of natural changes in hormone production and the structures of the uterus and ovaries of the female reproductive system that make pregnancy possible. The ovarian cycle controls the production and release of eggs and the cyclic release of estrogen and progesterone. The uterine cycle governs the preparation and maintenance of the lining of the uterus (womb) to receive a fertilized egg. These cycles are concurrent and coordinated, normally last between 21 and 35 days in adult women, with a median length of 28 days, and continue for about 30–45 years.
Luteinizing hormone is a hormone produced by gonadotropic cells in the anterior pituitary gland. The production of LH is regulated by gonadotropin-releasing hormone (GnRH) from the hypothalamus. In females, an acute rise of LH triggers ovulation and development of the corpus luteum. In males, where LH had also been called interstitial cell–stimulating hormone (ICSH), it stimulates Leydig cell production of testosterone. It acts synergistically with follicle-stimulating hormone (FSH).

Follicle-stimulating hormone (FSH) is a gonadotropin, a glycoprotein polypeptide hormone. FSH is synthesized and secreted by the gonadotropic cells of the anterior pituitary gland and regulates the development, growth, pubertal maturation, and reproductive processes of the body. FSH and luteinizing hormone (LH) work together in the reproductive system.
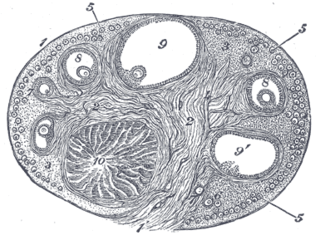
The corpus luteum is a temporary endocrine structure in female ovaries and is involved in the production of relatively high levels of progesterone and moderate levels of estradiol and inhibin A. It is the remains of the ovarian follicle that has released a mature ovum during a previous ovulation.
Gonadotropins are glycoprotein hormones secreted by gonadotropic cells of the anterior pituitary of vertebrates. This family includes the mammalian hormones follicle-stimulating hormone (FSH) and luteinizing hormone (LH), the placental/chorionic gonadotropins, human chorionic gonadotropin (hCG) and equine chorionic gonadotropin (eCG), as well as at least two forms of fish gonadotropins. These hormones are central to the complex endocrine system that regulates normal growth, sexual development, and reproductive function. LH and FSH are secreted by the anterior pituitary gland, while hCG and eCG are secreted by the placenta in pregnant humans and mares, respectively. The gonadotropins act on the gonads, controlling gamete and sex hormone production.

An ovarian follicle is a roughly spheroid cellular aggregation set found in the ovaries. It secretes hormones that influence stages of the menstrual cycle. At the time of puberty, women have approximately 200,000 to 300,000 follicles, each with the potential to release an egg cell (ovum) at ovulation for fertilization. These eggs are developed once every menstrual cycle with around 450-500 being ovulated during a woman's reproductive lifetime.

The corpus albicans is the regressed form of the corpus luteum. As the corpus luteum is being broken down by macrophages, fibroblasts lay down type I collagen, forming the corpus albicans. This process is called "luteolysis". The remains of the corpus albicans may persist as a scar on the surface of the ovary.

A granulosa cell or follicular cell is a somatic cell of the sex cord that is closely associated with the developing female gamete in the ovary of mammals.

In biology, folliculogenesis is the maturation of the ovarian follicle, a densely packed shell of somatic cells that contains an immature oocyte. Folliculogenesis describes the progression of a number of small primordial follicles into large preovulatory follicles that occurs in part during the menstrual cycle.

The follicular phase, also known as the preovulatory phase or proliferative phase, is the phase of the estrous cycle during which follicles in the ovary mature from primary follicle to a fully mature graafian follicle. It ends with ovulation. The main hormones controlling this stage are secretion of gonadotropin-releasing hormones, which are follicle-stimulating hormones and luteinising hormones. They are released by pulsatile secretion. The duration of the follicular phase can differ depending on the length of the menstrual cycle, while the luteal phase is usually stable, does not really change and lasts 14 days.
Follicular atresia is the breakdown of the ovarian follicles, which consist of an oocyte surrounded by granulosa cells and internal and external theca cells. It occurs continually throughout a woman’s life, as they are born with millions of follicles but will only ovulate around 400 times in their lifetime. Typically around 20 follicles mature each month but only a single follicle is ovulated; the follicle from which the oocyte was released becomes the corpus luteum. The rest undergo atresia.
Theca interna cells express receptors for luteinizing hormone (LH) to produce androstenedione, which via a few steps, gives the granulosa the precursor for estrogen manufacturing.
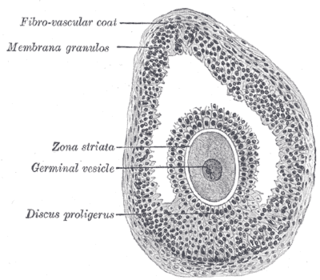
The cumulus oophorus,, is a cluster of cells that surround the oocyte both in the ovarian follicle and after ovulation. In the antral follicle, it may be regarded as an extension of the membrana granulosa. The innermost layer of these cells is the corona radiata.
The theca externa is the outer layer of the theca folliculi. It is derived from connective tissue, the cells resembling fibroblasts, and contains abundant collagen. During ovulation, the surge in luteinizing hormone increases cAMP which increases progesterone and PGF2α production. The PGF2α induces the contraction of the smooth muscle cells of the theca externa, increasing intrafollicular pressure. This aids in rupture of the mature oocyte, or immature oocyte at the germinal vesicle stage in the canine, along with plasmin and collagenase degradation of the follicle wall.

An antral follicle, also known as Graafian follicle and tertiary follicle, is an ovarian follicle during a certain latter stage of folliculogenesis.
Poor ovarian reserve is a condition of low fertility characterized by 1): low numbers of remaining oocytes in the ovaries or 2) possibly impaired preantral oocyte development or recruitment. Recent research suggests that premature ovarian aging and premature ovarian failure may represent a continuum of premature ovarian senescence. It is usually accompanied by high FSH levels.
Follicle-stimulating hormone (FSH) insensitivity, or ovarian insensitivity to FSH in females, also referable to as ovarian follicle hypoplasia or granulosa cell hypoplasia in females, is a rare autosomal recessive genetic and endocrine syndrome affecting both females and males, with the former presenting with much greater severity of symptomatology. It is characterized by a resistance or complete insensitivity to the effects of follicle-stimulating hormone (FSH), a gonadotropin which is normally responsible for the stimulation of estrogen production by the ovaries in females and maintenance of fertility in both sexes. The condition manifests itself as hypergonadotropic hypogonadism, reduced or absent puberty, amenorrhea, and infertility in females, whereas males present merely with varying degrees of infertility and associated symptoms.
Gonadotropin surge-attenuating factor (GnSAF) is a nonsteroidal ovarian hormone produced by the granulosa cells of small antral ovarian follicles in females. GnSAF is involved in regulating the secretion of luteinizing hormone (LH) from the anterior pituitary and the ovarian cycle. During the early to mid-follicular phase of the ovarian cycle, GnSAF acts on the anterior pituitary to attenuate LH release, limiting the secretion of LH to only basal levels. At the transition between follicular and luteal phase, GnSAF bioactivity declines sufficiently to permit LH secretion above basal levels, resulting in the mid-cycle LH surge that initiates ovulation. In normally ovulating women, the LH surge only occurs when the oocyte is mature and ready for extrusion. GnSAF bioactivity is responsible for the synchronised, biphasic nature of LH secretion.
Ovarian follicle dominance is the process where one or more follicles are selected per cycle to ovulate.