
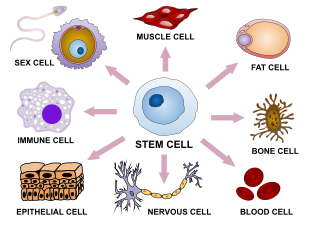
In multicellular organisms, stem cells are undifferentiated or partially differentiated cells that can differentiate into various types of cells and proliferate indefinitely to produce more of the same stem cell. They are the earliest type of cell in a cell lineage. They are found in both embryonic and adult organisms, but they have slightly different properties in each. They are usually distinguished from progenitor cells, which cannot divide indefinitely, and precursor or blast cells, which are usually committed to differentiating into one cell type.
Cellular differentiation is the process in which a stem cell changes from one type to a differentiated one. Usually, the cell changes to a more specialized type. Differentiation happens multiple times during the development of a multicellular organism as it changes from a simple zygote to a complex system of tissues and cell types. Differentiation continues in adulthood as adult stem cells divide and create fully differentiated daughter cells during tissue repair and during normal cell turnover. Some differentiation occurs in response to antigen exposure. Differentiation dramatically changes a cell's size, shape, membrane potential, metabolic activity, and responsiveness to signals. These changes are largely due to highly controlled modifications in gene expression and are the study of epigenetics. With a few exceptions, cellular differentiation almost never involves a change in the DNA sequence itself. However, metabolic composition does get altered quite dramatically where stem cells are characterized by abundant metabolites with highly unsaturated structures whose levels decrease upon differentiation. Thus, different cells can have very different physical characteristics despite having the same genome.
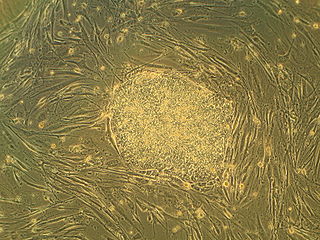
Embryonic stem cells (ESCs) are pluripotent stem cells derived from the inner cell mass of a blastocyst, an early-stage pre-implantation embryo. Human embryos reach the blastocyst stage 4–5 days post fertilization, at which time they consist of 50–150 cells. Isolating the inner cell mass (embryoblast) using immunosurgery results in destruction of the blastocyst, a process which raises ethical issues, including whether or not embryos at the pre-implantation stage have the same moral considerations as embryos in the post-implantation stage of development.

Oct-4, also known as POU5F1, is a protein that in humans is encoded by the POU5F1 gene. Oct-4 is a homeodomain transcription factor of the POU family. It is critically involved in the self-renewal of undifferentiated embryonic stem cells. As such, it is frequently used as a marker for undifferentiated cells. Oct-4 expression must be closely regulated; too much or too little will cause differentiation of the cells.
In biology, reprogramming refers to erasure and remodeling of epigenetic marks, such as DNA methylation, during mammalian development or in cell culture. Such control is also often associated with alternative covalent modifications of histones.

Jun dimerization protein 2 (JUNDM2) is a protein that in humans is encoded by the JDP2 gene. The Jun dimerization protein is a member of the AP-1 family of transcription factors.

Induced pluripotent stem cells are a type of pluripotent stem cell that can be generated directly from a somatic cell. The iPSC technology was pioneered by Shinya Yamanaka and Kazutoshi Takahashi in Kyoto, Japan, who together showed in 2006 that the introduction of four specific genes, collectively known as Yamanaka factors, encoding transcription factors could convert somatic cells into pluripotent stem cells. Shinya Yamanaka was awarded the 2012 Nobel Prize along with Sir John Gurdon "for the discovery that mature cells can be reprogrammed to become pluripotent."

Kruppel-like factor 4 is a member of the KLF family of zinc finger transcription factors, which belongs to the relatively large family of SP1-like transcription factors. KLF4 is involved in the regulation of proliferation, differentiation, apoptosis and somatic cell reprogramming. Evidence also suggests that KLF4 is a tumor suppressor in certain cancers, including colorectal cancer. It has three C2H2-zinc fingers at its carboxyl terminus that are closely related to another KLF, KLF2. It has two nuclear localization sequences that signals it to localize to the nucleus. In embryonic stem cells (ESCs), KLF4 has been demonstrated to be a good indicator of stem-like capacity. It is suggested that the same is true in mesenchymal stem cells (MSCs).

Shinya Yamanaka is a Japanese stem cell researcher and a Nobel Prize laureate. He is the former director of Center for iPS Cell Research and Application and a professor at the Institute for Frontier Medical Sciences at Kyoto University; as a senior investigator at the UCSF-affiliated Gladstone Institutes in San Francisco, California; and as a professor of anatomy at University of California, San Francisco (UCSF). Yamanaka is also a past president of the International Society for Stem Cell Research (ISSCR).
A mesenchymal–epithelial transition (MET) is a reversible biological process that involves the transition from motile, multipolar or spindle-shaped mesenchymal cells to planar arrays of polarized cells called epithelia. MET is the reverse process of epithelial–mesenchymal transition (EMT) and it has been shown to occur in normal development, induced pluripotent stem cell reprogramming, cancer metastasis and wound healing.

Cell potency is a cell's ability to differentiate into other cell types. The more cell types a cell can differentiate into, the greater its potency. Potency is also described as the gene activation potential within a cell, which like a continuum, begins with totipotency to designate a cell with the most differentiation potential, pluripotency, multipotency, oligopotency, and finally unipotency.

Forkhead box protein A2 (FOXA2), also known as hepatocyte nuclear factor 3-beta (HNF-3B), is a transcription factor that plays an important role during development, in mature tissues and, when dysregulated or mutated, also in cancer.
A list of examples of transdifferentiation:
A list of examples of in vivo transdifferentiation through transfection:
Induced stem cells (iSC) are stem cells derived from somatic, reproductive, pluripotent or other cell types by deliberate epigenetic reprogramming. They are classified as either totipotent (iTC), pluripotent (iPSC) or progenitor or unipotent – (iUSC) according to their developmental potential and degree of dedifferentiation. Progenitors are obtained by so-called direct reprogramming or directed differentiation and are also called induced somatic stem cells.

Glis1 is gene encoding a Krüppel-like protein of the same name whose locus is found on Chromosome 1p32.3. The gene is enriched in unfertilised eggs and embryos at the one cell stage and it can be used to promote direct reprogramming of somatic cells to induced pluripotent stem cells, also known as iPS cells. Glis1 is a highly promiscuous transcription factor, regulating the expression of numerous genes, either positively or negatively. In organisms, Glis1 does not appear to have any directly important functions. Mice whose Glis1 gene has been removed have no noticeable change to their phenotype.
Directed differentiation is a bioengineering methodology at the interface of stem cell biology, developmental biology and tissue engineering. It is essentially harnessing the potential of stem cells by constraining their differentiation in vitro toward a specific cell type or tissue of interest. Stem cells are by definition pluripotent, able to differentiate into several cell types such as neurons, cardiomyocytes, hepatocytes, etc. Efficient directed differentiation requires a detailed understanding of the lineage and cell fate decision, often provided by developmental biology.

Thomas Graf is a biologist at the Centre for Genomic Regulation (CRG) in Barcelona, Spain. He is a pioneer in cell reprogramming, showing that blood cells can be transdifferentiated by transcription factors. He is also known for his early work on oncogenes carried by retroviruses and oncogene cooperation in leukemia formation.
Transflammation describes the process by which innate immune response mechanisms affect the epigenetic plasticity of a cell during nuclear reprogramming. This phenomenon is essential in dedifferentiating a somatic cell to a pluripotent cell and also in transdifferentiating a terminally differentiated cell to another terminally differentiated cell.
Inner ear regeneration is the biological process by which the hair cells and supporting cells of the ear proliferate and regrow after hair cell injury. This process depends on communication between supporting cells and the brain. Because of the volatility of the inner ear's hair cells, regeneration is crucial to the functioning of the inner ear. It is also a limited process, which contributes to the irreversibility of hearing loss in humans and other mammals.











