
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily-related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. Coupling with G proteins, they are called seven-transmembrane receptors because they pass through the cell membrane seven times. Ligands can bind either to extracellular N-terminus and loops or to the binding site within transmembrane helices. They are all activated by agonists although a spontaneous auto-activation of an empty receptor can also be observed.
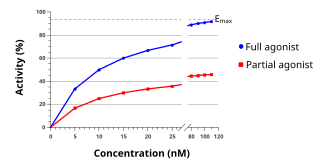
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist.
Functional selectivity is the ligand-dependent selectivity for certain signal transduction pathways relative to a reference ligand at the same receptor. Functional selectivity can be present when a receptor has several possible signal transduction pathways. To which degree each pathway is activated thus depends on which ligand binds to the receptor. Functional selectivity, or biased signaling, is most extensively characterized at G protein coupled receptors (GPCRs). A number of biased agonists, such as those at muscarinic M2 receptors tested as analgesics or antiproliferative drugs, or those at opioid receptors that mediate pain, show potential at various receptor families to increase beneficial properties while reducing side effects. For example, pre-clinical studies with G protein biased agonists at the μ-opioid receptor show equivalent efficacy for treating pain with reduced risk for addictive potential and respiratory depression. Studies within the chemokine receptor system also suggest that GPCR biased agonism is physiologically relevant. For example, a beta-arrestin biased agonist of the chemokine receptor CXCR3 induced greater chemotaxis of T cells relative to a G protein biased agonist.

The κ-opioid receptor or kappa opioid receptor, abbreviated KOR or KOP for its ligand ketazocine, is a G protein-coupled receptor that in humans is encoded by the OPRK1 gene. The KOR is coupled to the G protein Gi/G0 and is one of four related receptors that bind opioid-like compounds in the brain and are responsible for mediating the effects of these compounds. These effects include altering nociception, consciousness, motor control, and mood. Dysregulation of this receptor system has been implicated in alcohol and drug addiction.

G-protein-coupled receptor kinase 2 (GRK2) is an enzyme that in humans is encoded by the ADRBK1 gene. GRK2 was initially called Beta-adrenergic receptor kinase, and is a member of the G protein-coupled receptor kinase subfamily of the Ser/Thr protein kinases that is most highly similar to GRK3(βARK2).

The μ-opioid receptors (MOR) are a class of opioid receptors with a high affinity for enkephalins and beta-endorphin, but a low affinity for dynorphins. They are also referred to as μ(mu)-opioid peptide (MOP) receptors. The prototypical μ-opioid receptor agonist is morphine, the primary psychoactive alkaloid in opium and for which the receptor was named, with mu being the Greek letter m. It is an inhibitory G-protein coupled receptor that activates the Gi alpha subunit, inhibiting adenylate cyclase activity, lowering cAMP levels.
The δ-opioid receptor, also known as delta opioid receptor or simply delta receptor, abbreviated DOR or DOP, is an inhibitory 7-transmembrane G-protein coupled receptor coupled to the G protein Gi/G0 and has enkephalins as its endogenous ligands. The regions of the brain where the δ-opioid receptor is largely expressed vary from species model to species model. In humans, the δ-opioid receptor is most heavily expressed in the basal ganglia and neocortical regions of the brain.

Nalfurafine is an antipruritic that is marketed in Japan for the treatment of uremic pruritus in individuals with chronic kidney disease undergoing hemodialysis. It acts as a potent, selective, centrally-penetrant κ-opioid receptor (KOR) agonist, and is the first and currently the only selective KOR agonist to have been approved for clinical use. It has also been dubiously referred to as the "first non-narcotic opioid drug" in history.

Noribogaine, or 12-hydroxyibogamine, is the principal psychoactive metabolite of the oneirogen ibogaine. It is thought to be involved in the antiaddictive effects of ibogaine-containing plant extracts, such as Tabernanthe iboga.

G-protein-coupled receptor kinase 3 (GRK3) is an enzyme that in humans is encoded by the ADRBK2 gene. GRK3 was initially called Beta-adrenergic receptor kinase 2 (βARK-2), and is a member of the G protein-coupled receptor kinase subfamily of the Ser/Thr protein kinases that is most highly similar to GRK2.

Sacubitril/valsartan, sold under the brand name Entresto, is a fixed-dose combination medication for use in heart failure. It consists of the neprilysin inhibitor sacubitril and the angiotensin receptor blocker valsartan. It is recommended for use as a replacement for an ACE inhibitor or an angiotensin receptor blocker in people with heart failure with reduced ejection fraction.

Mitragynine pseudoindoxyl is a rearrangement product of 7-hydroxymitragynine. It is an analgesic being more potent than morphine.

Oliceridine, sold under the brand name Olinvyk, is an opioid medication that is used for the treatment of moderate to severe acute pain in adults. It is given by intravenous (IV) injection.
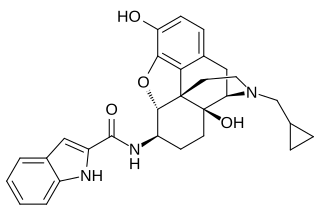
N-2′-Indolylnaltrexamine (INTA) is an opioid and derivative of β-naltrexamine. This molecule is loosely derived from the classical opioid morphine. This experimental drug candidate is under development as a κ-opioid receptor agonist for pain management with fewer adverse side effects. Preclinical study in mice showed potent analgesic effects with no tolerance or dependence. The mice also showed no adverse effects in the conditioned place aversion assay.

PZM21 is an experimental opioid analgesic drug that is being researched for the treatment of pain. It is claimed to be a functionally selective μ-opioid receptor agonist which produces μ-opioid receptor mediated G protein signaling, with potency and efficacy similar to morphine, but with less β-arrestin 2 recruitment. However, recent reports highlight that this might be due to its low intrinsic efficacy, rather than functional selectivity or 'G protein bias' as initially reported. In tests on mice, PZM21 was slightly less potent than morphine or TRV130 as an analgesic, but also had significantly reduced adverse effects, with less constipation than morphine, and very little respiratory depression, even at high doses. This research was described as a compelling example of how modern high-throughput screening techniques can be used to discover new chemotypes with specific activity profiles, even at targets such as the μ-opioid receptor which have already been thoroughly investigated. More recent research has suggested however that at higher doses, PZM21 is capable of producing classic opioid side effects such as respiratory depression and development of tolerance and may have only limited functional selectivity.

Mitragynine is an indole-based alkaloid and the most abundant active alkaloid in the Southeast Asian plant Mitragyna speciosa, commonly known as kratom. The total alkaloid concentration in dried leaves ranges from 0.5 to 1.5%. In Thai varieties, mitragynine is the most abundant component while 7-hydroxymitragynine is a minor constituent. In Malaysian kratom varieties, mitragynine is present at lower concentration. Such preparations are orally consumed and typically involve dried kratom leaves which are brewed into tea or ground and placed into capsules. Mitragynine consumption for medicinal and recreation purposes dates back centuries, although early use was primarily limited to Southeast Asian countries such as Indonesia and Thailand where the plant grows indigenously. Recently, mitragynine use has spread throughout Europe and the Americas as both a recreational and medicinal drug. While research into the effects of kratom have begun to emerge, investigations on the active compound mitragynine are less common.
Sadashiva "Sadu" Karnik is an Indian-born American molecular biologist who is a Professor in the Molecular Medicine Department of Cleveland Clinic Lerner College of Medicine at Case Western Reserve University. He is also head of the Karnik-lab at the Lerner Research Institute of Cleveland Clinic.

SR-17018 is a drug which acts as a biased agonist at the μ-opioid receptor, selective for activation of the G-protein signalling pathway over β-arrestin 2 recruitment. In animal studies it produces analgesic effects but with less respiratory depression and development of tolerance than conventional opioids.

TRV734 is a drug developed by Trevena Inc which acts as a biased agonist at the μ-opioid receptor, selective for activation of the G-protein signalling pathway over β-arrestin 2 recruitment. It is closely related to oliceridine and has a similar pharmacological profile, but unlike oliceridine which has to be injected, TRV734 is suitable to be administered orally.

SHR9352 is a drug which acts as a potent and selective biased agonist at the μ-opioid receptor, selective for activation of the G-protein signalling pathway over β-arrestin 2 recruitment. It was structurally derived from oliceridine by replacing the benzylic side chain with a cyclised group, although only some compounds in the series retained the desired biased agonist profile, with some derivatives such as compound 12 being potent, unbiased μ-opioid full agonists.

















