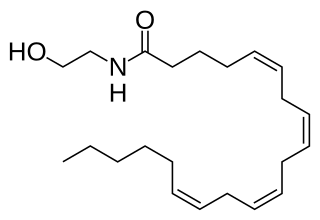
Anandamide (ANA), also known as N-arachidonoylethanolamine (AEA), an N-acylethanolamine (NAE), is a fatty acid neurotransmitter. Anandamide was the first endocannabinoid to be discovered: it participates in the body's endocannabinoid system by binding to cannabinoid receptors, the same receptors that the psychoactive compound THC in cannabis acts on. Anandamide is found in nearly all tissues in a wide range of animals. Anandamide has also been found in plants, including small amounts in chocolate. The name 'anandamide' is taken from the Sanskrit word ananda, which means "joy, bliss, delight", plus amide.

URB754 was originally reported by Piomelli et al. to be a potent, noncompetitive inhibitor of monoacylglycerol lipase (MGL). However, recent studies have shown that URB754 failed to inhibit recombinant MGL, and brain FAAH activity was also resistant to URB754. In a later study by Piomelli et al., the MGL-inhibitory activity attributed to URB754 is in fact due to a chemical impurity present in the commercial sample, identified as bis(methylthio)mercurane.

Monoacylglycerol lipase is an enzyme that, in humans, is encoded by the MGLL gene. MAGL is a 33-kDa, membrane-associated member of the serine hydrolase superfamily and contains the classical GXSXG consensus sequence common to most serine hydrolases. The catalytic triad has been identified as Ser122, His269, and Asp239.

Fatty-acid amide hydrolase 1 or FAAH-1(EC 3.5.1.99, oleamide hydrolase, anandamide amidohydrolase) is a member of the serine hydrolase family of enzymes. It was first shown to break down anandamide (AEA), an N-acylethanolamine (NAE) in 1993. In humans, it is encoded by the gene FAAH. FAAH also regulate the contents of NAE's in Dictyostelium discoideum, as they modulate their NAE levels in vivo through the use of a semispecific FAAH inhibitor.

AM404, also known as N-arachidonoylphenolamine, is an active metabolite of paracetamol (acetaminophen), responsible for all or part of its analgesic action and anticonvulsant effects. Chemically, it is the amide formed from 4-aminophenol and arachidonic acid.
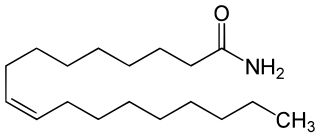
Oleamide is an organic compound with the formula CH3(CH2)7CH=CH(CH2)7CONH2. It is the amide derived from the fatty acid oleic acid. It is a colorless waxy solid and occurs in nature. Sometimes labeled as a fatty acid primary amide (FAPA), it is biosynthesized from N-oleoylglycine.

Methoxy arachidonyl fluorophosphonate, commonly referred as MAFP, is an irreversible active site-directed enzyme inhibitor that inhibits nearly all serine hydrolases and serine proteases. It inhibits phospholipase A2 and fatty acid amide hydrolase with special potency, displaying IC50 values in the low-nanomolar range. In addition, it binds to the CB1 receptor in rat brain membrane preparations (IC50 = 20 nM), but does not appear to agonize or antagonize the receptor, though some related derivatives do show cannabinoid-like properties.

JZL184 is an irreversible inhibitor for monoacylglycerol lipase (MAGL), the primary enzyme responsible for degrading the endocannabinoid 2-arachidonoylglycerol (2-AG). It displays high selectivity for MAGL over other brain serine hydrolases, including the anandamide-degrading enzyme fatty acid amide hydrolase (FAAH), thereby making it a useful tool for studying the effects of endogenous 2-AG signaling, in vivo. Administration of JZL184 to mice was reported to cause dramatic elevation of brain 2-AG leading to several cannabinoid-related behavioral effects.
Palmitoylethanolamide (PEA) is an endogenous fatty acid amide, and lipid modulator PEA has been studied in in vitro and in vivo systems using exogenously added or dosed compound; there is evidence that it binds to a nuclear receptor, through which it exerts a variety of biological effects, some related to chronic inflammation and pain.

URB602 is a compound that has been found to inhibit hydrolysis of monoacyl glycerol compounds, such as 2-arachidonoylglycerol (2-AG) and 2-oleoylglycerol (2-OG). It was first described in 2003. A study performed in 2005 found that the compound had specificity for metabolizing 2-AG over anandamide in rat brain presumably by inhibiting the enzyme monoacylglycerol lipase (MAGL), which is the primary metabolic enzyme of 2-AG. However, subsequent studies have shown that URB602 lacks specificity for MAGL inhibition in vitro.

JZL195 is a potent inhibitor of both fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), the primary enzymes responsible for degrading the endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG), respectively.

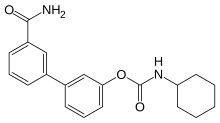
LY-2183240 is a drug which acts both as a potent inhibitor of the reuptake of the endocannabinoid anandamide and as an inhibitor of fatty acid amide hydrolase (FAAH), the primary enzyme responsible for degrading anandamide. This leads to markedly elevated anandamide levels in the brain, and LY-2183240 has been shown to produce both analgesic and anxiolytic effects in animal models. While LY-2183240 is a potent inhibitor of FAAH, it has relatively poor selectivity and also inhibits several other enzyme side targets. Consequently, it was never developed for clinical use, though it remains widely used in research, and has also been sold as a designer drug.
IDFP is an organophosphorus compound related to the nerve agent sarin.
The endocannabinoid transporters (eCBTs) are transport proteins for the endocannabinoids. Most neurotransmitters are water-soluble and require transmembrane proteins to transport them across the cell membrane. The endocannabinoids on the other hand, are non-charged lipids that readily cross lipid membranes. However, since the endocannabinoids are water immiscible, protein transporters have been described that act as carriers to solubilize and transport the endocannabinoids through the aqueous cytoplasm. These include the heat shock proteins (Hsp70s) and fatty acid-binding proteins for anandamide (FABPs). FABPs such as FABP1, FABP3, FABP5, and FABP7 have been shown to bind endocannabinoids. FABP inhibitors attenuate the breakdown of anandamide by the enzyme fatty acid amide hydrolase (FAAH) in cell culture. One of these inhibitors (SB-FI-26), isolated from a virtual library of a million compounds, belongs to a class of compounds that act as an anti-nociceptive agent with mild anti-inflammatory activity in mice. These truxillic acids and their derivatives have been known to have anti-inflammatory and anti-nociceptive effects in mice and are active components of a Chinese herbal medicine used to treat rheumatism and pain in human. The blockade of anandamide transport may, at least in part, be the mechanism through which these compounds exert their anti-nociceptive effects.
4-Nonylphenylboronic acid is a potent and selective inhibitor of the enzyme fatty acid amide hydrolase (FAAH), with an IC50 of 9.1nM, and 870x selectivity for FAAH over the related enzyme MAGL, which it inhibits with an IC50 of 7900nM. It is also a weaker inhibitor of the enzymes endothelial lipase and lipoprotein lipase, with IC50 values of 100 nM and 1400 nM respectively.
Daniele Piomelli is an Italian-born American scientist. He studied neuroscience in New York City, with James H. Schwartz and Eric R. Kandel at Columbia University College of Physicians and Surgeons and later with Paul Greengard at the Rockefeller University. Two of his mentors received in 2000 the Nobel Prize for their contributions to medicine. After working at the INSERM in Paris (1990-1995) and at the Neurosciences Institute in La Jolla (1995-1998) with Nobel Prize winner Gerald Edelman, he joined the University of California Irvine School of Medicine, where he is now Louise Turner Arnold Chair in Neurosciences and Professor of Anatomy and Neurobiology, Pharmacology and Biological Chemistry. He is also founding director of the department of Drug Discovery and Development (D3) at the Istituto Italiano di Tecnologia in Genova, Italy. He is also the editor of Cannabis and Cannabinoid Research and a board member of the non-profit International Association for Cannabinoid Medicines.
N-acylethanolamine acid amide hydrolase (NAAA) EC 3.5.1.- is a member of the choloylglycine hydrolase family, a subset of the N-terminal nucleophile hydrolase superfamily. NAAA has a molecular weight of 31 kDa. The activation and inhibition of its catalytic site is of medical interest as a potential treatment for obesity and chronic pain. While it was discovered within the last decade, its structural similarity to the more familiar acid ceramidase (AC) and functional similarity to fatty acid amide hydrolase (FAAH) allow it to be studied extensively.
PF-3845 is a selective inhibitor of fatty acid amide hydrolase. It results in increased levels of anandamide and results in cannabinoid receptor-based effects. It has anti-inflammatory action in mice colitis models. Antidiarrheal and antinociceptive effects were also seen in mouse models of pain.

MK-4409 is an experimental drug which acts as a potent and selective inhibitor of the enzyme fatty acid amide hydrolase (FAAH), with an IC50 of 11 nM, and both analgesic and antiinflammatory effects in animal studies. It was studied for the treatment of neuropathic pain and progressed to early stage human clinical trials by 2009.
An endocannabinoid enhancer (eCBE) is a type of cannabinoidergic drug that enhances the activity of the endocannabinoid system by increasing extracellular concentrations of endocannabinoids. Examples of different types of eCBEs include fatty acid amide hydrolase (FAAH) inhibitors, monoacylglycerol lipase (MAGL) inhibitors, and endocannabinoid transporter (eCBT) inhibitors. An example of an actual eCBE is AM404, the active metabolite of the analgesic paracetamol and a dual FAAH inhibitor and eCBRI.