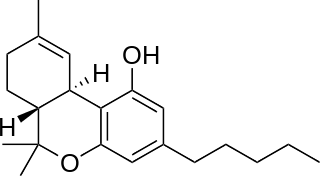
Tetrahydrocannabinol (THC) is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term THC usually refers to the Delta-9-THC isomer with chemical name (−)-trans-Δ9-tetrahydrocannabinol. Like most pharmacologically active secondary metabolites of plants, THC is a lipid found in cannabis, assumed to be involved in the plant's evolutionary adaptation, putatively against insect predation, ultraviolet light, and environmental stress.

Cannabinoids are compounds found in the cannabis plant or synthetic compounds that can interact with the endocannabinoid system. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (Delta-9-THC), the primary intoxicating compound in cannabis. Cannabidiol (CBD) is another major constituent of the some cannabis plants. At least 113 distinct cannabinoids have been isolated from cannabis. It was reported in 2020 that cannabinoids can be found in other plants such as rhododendron, licorice and liverwort, and earlier in Echinacea.

Cannabis sativa is an annual herbaceous flowering plant indigenous to Eastern Asia, but now of cosmopolitan distribution due to widespread cultivation. It has been cultivated throughout recorded history, used as a source of industrial fiber, seed oil, food, recreation, religious and spiritual moods and medicine. Each part of the plant is harvested differently, depending on the purpose of its use. The species was first classified by Carl Linnaeus in 1753. The word sativa means "things that are cultivated."
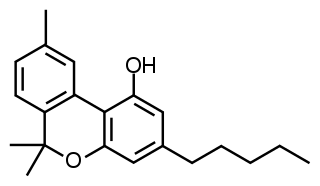
Cannabinol (CBN) is a non-psychoactive cannabinoid found in trace amounts from Cannabis. CBN is mostly found in cannabis that is aged and stored, and is derived from the plant's main psychoactive chemical, tetrahydrocannabinol (THC).

Cannabidiol (CBD) is a phytocannabinoid discovered in 1940. It is one of 113 identified cannabinoids in cannabis plants, along with tetrahydrocannabinol (THC), and accounts for up to 40% of the plant's extract. As of 2019, clinical research on CBD included studies related to anxiety, cognition, movement disorders, and pain, but there is insufficient high-quality evidence that cannabidiol is effective for these conditions.

Cannabivarin (CBV), also known as cannabivarol, is considered a non-psychoactive cannabinoid — it does not produce the euphoric side effects found in THC. Minor amounts of CBV are found in the hemp plant Cannabis sativa. It is an analog of cannabinol (CBN) with the side chain shortened by two methylene bridges (-CH2-). CBV is an oxidation product of tetrahydrocannabivarin (THCV, THV).

Cannabidivarin (CBDV, GWP42006) is a non-psychoactive cannabinoid found in Cannabis. It is a homolog of cannabidiol (CBD), with the side-chain shortened by two methylene bridges (CH2 units).

Cannabis tea is a cannabis-infused drink prepared by steeping various parts of the cannabis plant in hot or cold water. Cannabis tea is commonly recognized as an alternative form of preparation and consumption of the cannabis plant, more popularly known as marijuana, pot, or weed. This plant has long been recognized as an herbal medicine employed by health professionals worldwide to ease symptoms of disease, as well as a psychoactive drug used recreationally and in spiritual traditions. Though less commonly practiced than popular methods like smoking or consuming edibles, drinking cannabis tea can produce comparable physical and mental therapeutic effects. Such effects are largely attributed to the THC content of the tea, levels of which are drastically dependent on individual preparation techniques involving volume, amount of cannabis, and boiling time. Also in common with these administration forms of cannabis is the heating component performed before usage. Due to the rather uncommon nature of this particular practice of cannabis consumption in modern times, the research available on the composition of cannabis tea is limited and based broadly around what is known of cannabis as it exists botanically.

Cannabigerol (CBG) is one of more than 120 identified cannabinoid compounds found in the plant genus Cannabis. Cannabigerol is the decarboxylated form of cannabigerolic acid, the parent molecule from which other cannabinoids are synthesized. Cannabigerol is a minor constituent of cannabis. During plant growth, most of the cannabigerol is converted into other cannabinoids, primarily tetrahydrocannabinol (THC) or cannabidiol (CBD), leaving about 1% cannabigerol in the plant.
Cannabichromene (CBC), also called cannabichrome, cannanbichromene, pentylcannabichromene or cannabinochromene, is an anti-inflammatory which may contribute to the pain-killing effect of cannabis. It is one of the hundreds of cannabinoids found in the Cannabis plant, and is therefore a phytocannabinoid. It bears structural similarity to the other natural cannabinoids, including tetrahydrocannabinol (THC), tetrahydrocannabivarin (THCV), cannabidiol (CBD), and cannabinol (CBN), among others. CBC and its derivatives are as abundant as cannabinols in cannabis. It is not scheduled by the Convention on Psychotropic Substances. It is more common in tropical cannabis varieties.

Cannabis strains are either pure or hybrid varieties of the plant genus Cannabis, which encompasses the species C. sativa, C. indica, and C. ruderalis.

The International Nonproprietary Name Dronabinol, also known as delta-9-tetrahydrocannabinol, or under the trade names Marinol, Syndros, Reduvo and Adversa, is a generic name for the molecule of delta-9-tetrahydrocannabinol in the pharmaceutical context. It has indications as an appetite stimulant, antiemetic, and sleep apnea reliever and is approved by the FDA as safe and effective for HIV/AIDS-induced anorexia and chemotherapy-induced nausea and vomiting only.

Tetrahydrocannabinolic acid (THCA) synthase is an enzyme responsible for catalyzing the formation of THCA from cannabigerolic acid (CBGA). THCA is the direct precursor of tetrahydrocannabinol (THC), the principal psychoactive component of cannabis, which is produced from various strains of Cannabis sativa. Therefore, THCA synthase is considered to be a key enzyme controlling cannabis psychoactivity. Polymorphisms of THCA synthase result in varying levels of THC in Cannabis plants, resulting in "drug-type" and "fiber-type" C. sativa varieties.
The entourage effect is a proposed mechanism by which cannabis compounds other than tetrahydrocannabinol (THC) act synergistically with it to modulate the overall psychoactive effects of the plant.

The following outline is provided as an overview of and topical guide to the plant Cannabis sativa and its relatives Cannabis indica and Cannabis ruderalis, the drug cannabis (drug) and the industrial product hemp.
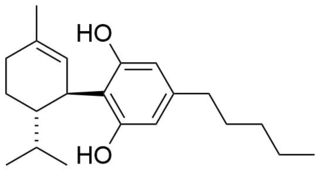
8,9-Dihydrocannabidiol (H2CBD) is a synthetic cannabinoid that is closely related to cannabidiol (CBD) itself. It was shown to have anti-seizure activity essentially identical to that of CBD in tests with rats. It may have certain advantages over CBD, in that it is fully synthetic, inexpensive to produce, and it is not a scheduled drug. In addition, there is no path to synthesize the psychoactive substance tetrahydrocannabinol (THC) from H2CBD. CBD has been shown to convert to some extent to THC in the gastric tract, and the deliberate laboratory conversion of CBD to THC is straightforward. H2CBD has therefore been studied for its potential use as an alternative to CBD in terms of its lack of abuse liability and absence of psychotropic effects.

Delta-8-tetrahydrocannabinol is a psychoactive cannabinoid found in the Cannabis plant. It is an isomer of delta-9-tetrahydrocannabinol, the compound commonly known as THC. ∆8-THC is under preliminary research for its biological properties.
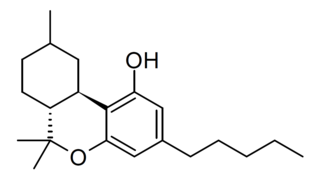
Hexahydrocannabinol (HHC) is a hydrogenated derivative of tetrahydrocannabinol. It is a naturally occurring phytocannabinoid that has rarely been identified as a trace component in Cannabis sativa, but can also be produced synthetically by hydrogenation of cannabis extracts. HHC was first synthesized in 1947 by Roger Adams using natural THC found in Cannabis sativa. Several research groups have successfully synthesized (+)-HHC and (-)-HHC using citronellal and olivetol, as well as other related compounds. While similar compounds have previously been identified in cannabis, hexahydrocannabinol itself has rarely been isolated from the plant. The de Las Heras group in 2020 took lipid extract from Cannabis sativa seeds and discovered 43 cannabinoids in the crude extract; one of them being hexahydrocannabinol. It has two diastereomers at the methyl (9) position. HHC is typically made from CBD, Δ8-THC, or Δ9-THC. There are no double bonds in the cyclohexyl ring like D8/D9 have—they have been removed from the structure and hydrogens have been added to the compound.Similar structural analogs of HHC has been demonstrated to bind to the CB1 receptor and produces cannabinoid effects in animals, with the 9β-HHC enantiomer being much more active than 9α-HHC. While HHC has been shown to bind to the CB1 receptor, it binds with weaker affinity than THC, which has typically been an indication that it is not as intoxicating as a typical THC. Since HHC is found naturally in the cannabis plant, humans have likely been unknowingly consuming small amounts of this cannabinoid for centuries.

Cannabimovone (CBM) is a phytocannabinoid first isolated from a non-psychoactive strain of Cannabis sativa in 2010, which is thought to be a rearrangement product of cannabidiol. It lacks affinity for cannabinoid receptors, but acts as an agonist at both TRPV1 and PPARγ.















