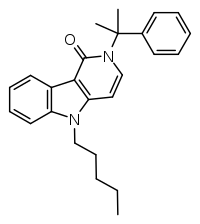
MN 18 is an indazole-based synthetic cannabinoid that is an agonist for the cannabinoid receptors, with Ki values of 45.72 nM at CB1 and 11.098 nM at CB2 and EC50 values of 2.028 nM at CB1 and 1.233 nM at CB2, and has been sold online as a designer drug. It is the indazole core analogue of NNE1. Given the known metabolic liberation (and presence as an impurity) of amantadine in the related compound APINACA, it is suspected that metabolic hydrolysis of the amide group of MN-18 may release 1-naphthylamine, a known carcinogen. MN-18 metabolism has been described in literature.
MN-25 (UR-12) is a drug invented by Bristol-Myers Squibb, that acts as a reasonably selective agonist of peripheral cannabinoid receptors. It has moderate affinity for CB2 receptors with a Ki of 11 nM, but 22x lower affinity for the psychoactive CB1 receptors with a Ki of 245 nM. The indole 2-methyl derivative has the ratio of affinities reversed however, with a Ki of 8 nM at CB1 and 29 nM at CB2, which contrasts with the usual trend of 2-methyl derivatives having increased selectivity for CB2 (cf. JWH-018 vs JWH-007, JWH-081 vs JWH-098).

SDB-006 is a drug that acts as a potent agonist for the cannabinoid receptors, with an EC50 of 19 nM for human CB2 receptors, and 134 nM for human CB1 receptors. It was discovered during research into the related compound SDB-001 which had been sold illicitly as "2NE1". SDB-006 metabolism has been described in literature.

AB-CHFUPYCA is a compound that was first identified as a component of synthetic cannabis products in Japan in 2015. The name "AB-CHFUPYCA" is an acronym of its systematic name N-(1-Amino-3-methyl-1-oxoButan-2-yl)-1-(CycloHexylmethyl)-3-(4-FlUorophenyl)-1H-PYrazole-5-CarboxAmide. There are two known regioisomers of AB-CHFUPYCA: 3,5-AB-CHMFUPPYCA (pictured) and 5,3-AB-CHMFUPPYCA. The article[1] refers to both 3,5-AB-CHMFUPPYCA and 5,3-AB-CHMFUPPYCA as AB-CHMFUPPYCA isomers, so AB-CHMFUPPYCA and AB-CHFUPYCA are not names for a unique chemical structure.
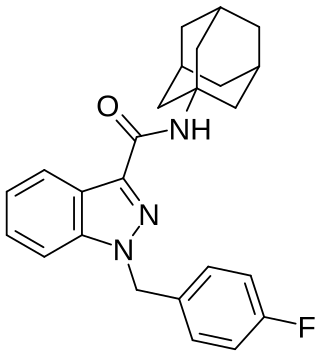
FUB-APINACA (also known as A-FUBINACA according to the EMCCDA framework for naming synthetic cannabinoids and FUB-AKB48) is an indazole-based synthetic cannabinoid that is presumed to be a potent agonist of the CB1 receptor and has been sold online as a designer drug. It is an analog of APINACA and 5F-APINACA where the pentyl chain has been replaced with fluorobenzyl.
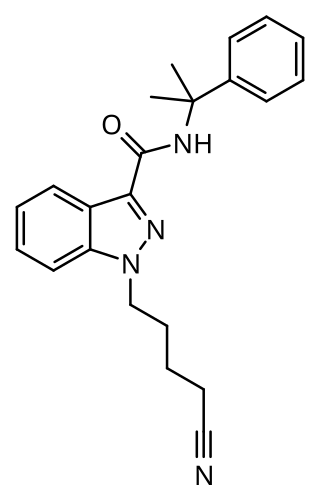
CUMYL-4CN-BINACA (also known as CUMYL-CYBINACA or SGT-78) is an indazole-3-carboxamide based synthetic cannabinoid that has been sold online as a designer drug. It is a potent agonist for cannabinoid receptors CB1 and CB2, with in vitro EC50 values of 0.58 nM and 6.12 nM, respectively. In mice, CUMYL-4CN-BINACA produces hypothermic and pro-convulsant effects via the CB1 receptor, and anecdotal reports suggest it has an active dose of around 0.1 mg in humans.

5F-CUMYL-P7AICA is a pyrrolo[2,3-b]pyridine-3-carboxamide based synthetic cannabinoid that has been sold as a designer drug. It was first identified by the EMCDDA in February 2015.

5F-MDMB-PICA is a designer drug and synthetic cannabinoid. In 2018, it was the fifth-most common synthetic cannabinoid identified in drugs seized by the Drug Enforcement Administration.

5F-EDMB-PINACA is a designer drug and synthetic cannabinoid. In 2018, it was the fourth-most common synthetic cannabinoid identified in drugs seized by the Drug Enforcement Administration.

MDMB-4en-PINACA is an indazole-based synthetic cannabinoid that has been sold online as a designer drug. MDMB-4en-PINACA was first identified in Europe in 2017. In 2021, MDMB-4en-PINACA was the most common synthetic cannabinoid identified by the Drug Enforcement Administration in the United States. MDMB-4en-PINACA differs from 5F-MDMB-PINACA due to replacement of 5-fluoropentyl with a pent-4-ene moiety (4-en).
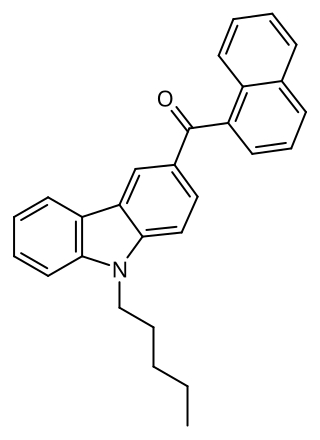
EG-018 is a carbazole-based synthetic cannabinoid that has been sold online as a designer drug. It acts as a partial agonist of the CB1 and CB2 receptor, with reasonably high binding affinity, but low efficacy in terms of inducing a signaling response.

5F-CUMYL-PEGACLONE (5F-SGT-151, SGT-269) is a gamma-carboline based synthetic cannabinoid that has been sold as a designer drug, first being identified in Germany in 2017. It acts as a potent full agonist of the CB1 receptor. It appears to be more toxic than related compounds such as CUMYL-PEGACLONE, and has been linked to numerous serious adverse reactions, some fatal.

CUMYL-CH-MEGACLONE is a gamma-carboline based synthetic cannabinoid receptor agonist that has been sold as a designer drug, first being identified in Hungary in December 2018.
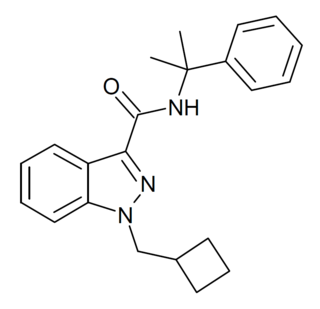
CUMYL-CBMINACA (SGT-277) is an indazole-3-carboxamide based synthetic cannabinoid receptor agonist that has been sold as a designer drug, first being identified in Germany in February 2020. It is illegal in Finland.

CUMYL-CB-MEGACLONE is a gamma-carboline based synthetic cannabinoid receptor agonist that has been sold as a designer drug, first being identified in Hungary in April 2020.

CUMYL-CBMICA (SGT-280) is an indole-3-carboxamide based synthetic cannabinoid receptor agonist which has been sold as a designer drug, first being identified in Germany in August 2019. Since the structure fell outside the German drug analogue law provisions at the time, an amendment was made to the law to expand the relevant definition, which came into effect in April 2020. It has been shown to act as a CB1 receptor agonist with an EC50 of 62.9nM.
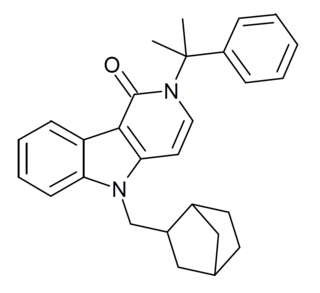
Cumyl-BC-HpMeGaClone-221 is a gamma-carboline derivative which is a synthetic cannabinoid that has been sold as a designer drug. It was first identified in Germany in September 2020.

ADB-BINACA is a cannabinoid designer drug that has been found as an ingredient in some synthetic cannabis products. It was originally developed by Pfizer as a potential analgesic, and is a potent agonist of the CB1 receptor with a binding affinity (Ki) of 0.33 nM and an EC50 of 14.7 nM.

ADB-4en-PINACA is a cannabinoid designer drug that has been found as an ingredient in some synthetic cannabis products, first appearing in early 2021. It is a reasonably potent cannabinoid agonist in vitro but has not been so widely sold as related compounds such as ADB-PINACA and MDMB-4en-PINACA.

BMS-F is a chemical from the aminoalkylindole family invented by Bristol-Myers Squibb around 1999, that acts as a potent and selective agonist for the cannabinoid receptor CB2, with a Ki of 8 nM at CB2 and 500x selectivity over the related CB1 receptor. It has antiinflammatory effects and inhibits release of TNF-α.