
The cerebellum is a major feature of the hindbrain of all vertebrates. Although usually smaller than the cerebrum, in some animals such as the mormyrid fishes it may be as large as or even larger. In humans, the cerebellum plays an important role in motor control. It may also be involved in some cognitive functions such as attention and language as well as emotional control such as regulating fear and pleasure responses, but its movement-related functions are the most solidly established. The human cerebellum does not initiate movement, but contributes to coordination, precision, and accurate timing: it receives input from sensory systems of the spinal cord and from other parts of the brain, and integrates these inputs to fine-tune motor activity. Cerebellar damage produces disorders in fine movement, equilibrium, posture, and motor learning in humans.

A dendritic spine is a small membranous protrusion from a neuron's dendrite that typically receives input from a single axon at the synapse. Dendritic spines serve as a storage site for synaptic strength and help transmit electrical signals to the neuron's cell body. Most spines have a bulbous head, and a thin neck that connects the head of the spine to the shaft of the dendrite. The dendrites of a single neuron can contain hundreds to thousands of spines. In addition to spines providing an anatomical substrate for memory storage and synaptic transmission, they may also serve to increase the number of possible contacts between neurons. It has also been suggested that changes in the activity of neurons have a positive effect on spine morphology.
Synaptogenesis is the formation of synapses between neurons in the nervous system. Although it occurs throughout a healthy person's lifespan, an explosion of synapse formation occurs during early brain development, known as exuberant synaptogenesis. Synaptogenesis is particularly important during an individual's critical period, during which there is a certain degree of synaptic pruning due to competition for neural growth factors by neurons and synapses. Processes that are not used, or inhibited during their critical period will fail to develop normally later on in life.

Basket cells are inhibitory GABAergic interneurons of the brain, found throughout different regions of the cortex and cerebellum.

Purkinje cells, or Purkinje neurons, are a class of GABAergic inhibitory neurons located in the cerebellum. They are named after their discoverer, Czech anatomist Jan Evangelista Purkyně, who characterized the cells in 1839.
A basal dendrite is a dendrite that emerges from the base of a pyramidal cell that receives information from nearby neurons and passes it to the soma, or cell body. Due to their direct attachment to the cell body itself, basal dendrites are able to deliver strong depolarizing currents and therefore have a strong effect on action potential output in neurons. The physical characteristics of basal dendrites vary based on their location and species that they are found in. For example, the basal dendrites of humans are overall found to be the most intricate and spine-dense, as compared to other species such as Macaques. It is also observed that basal dendrites of the prefrontal cortex are larger and more complex in comparison to the smaller and simpler dendrites that can be seen within the visual cortex. Basal dendrites are capable of vast amounts of analog computing, which is responsible for many of the different nonlinear responses of modulating information in the neocortex. Basal dendrites additionally exist in dentate granule cells for a limited time before removal via regulatory factors. This removal usually occurs before the cell reaches adulthood, and is thought to be regulated through both intracellular and extracellular signals. Basal dendrites are part of the more overarching dendritic tree present on pyramidal neurons. They, along with apical dendrites, make up the part of the neuron that receives most of the electrical signaling. Basal dendrites have been found to be involved mostly in neocortical information processing.

Stellate cells are any neuron in the central nervous system that have a star-like shape formed by dendritic processes radiating from the cell body. Many Stellate cells are GABAergic and are located in the molecular layer of the cerebellum. Stellate cells are derived from dividing progenitors in the white matter of postnatal cerebellum. Dendritic trees can vary between neurons. There are two types of dendritic trees in the cerebral cortex, which include pyramidal cells, which are pyramid shaped and stellate cells which are star shaped. Dendrites can also aid neuron classification. Dendrites with spines are classified as spiny, those without spines are classified as aspinous. Stellate cells can be spiny or aspinous, while pyramidal cells are always spiny. Most common stellate cells are the inhibitory interneurons found within the upper half of the molecular layer in the cerebellum. Cerebellar stellate cells synapse onto the dendritic arbors of Purkinje cells and send inhibitory signals. Stellate neurons are sometimes found in other locations in the central nervous system; cortical spiny stellate cells are found in layer IVC of the V1 region in the visual cortex. In the somatosensory barrel cortex of mice and rats, glutamatergic (excitatory) spiny stellate cells are organized in the barrels of layer 4. They receive excitatory synaptic fibres from the thalamus and process feed forward excitation to 2/3 layer of V1 visual cortex to pyramidal cells. Cortical spiny stellate cells have a 'regular' firing pattern. Stellate cells are chromophobes, that is cells that does not stain readily, and thus appears relatively pale under the microscope.

In neuroscience, Golgi cells are inhibitory interneurons found within the granular layer of the cerebellum. They were first identified as inhibitory by Eccles et al. in 1964. It was also the first example of an inhibitory feed back network, where the inhibitory interneuron was identified anatomically. These cells synapse onto the dendrite of granule cells and unipolar brush cells. They receive excitatory input from mossy fibres, also synapsing on granule cells, and parallel fibers, which are long granule cell axons. Thereby this circuitry allows for feed-forward and feed-back inhibition of granule cells.

The dorsal cochlear nucleus, is a cortex-like structure on the dorso-lateral surface of the brainstem. Along with the ventral cochlear nucleus (VCN), it forms the cochlear nucleus (CN), where all auditory nerve fibers from the cochlea form their first synapses.
The synaptotropic hypothesis, also called the synaptotrophic hypothesis, is a neurobiological hypothesis of neuronal growth and synapse formation. The hypothesis was first formulated by J.E. Vaughn in 1988, and remains a focus of current research efforts. The synaptotropic hypothesis proposes that input from a presynaptic to a postsynaptic cell eventually can change the course of synapse formation at dendritic and axonal arbors. This synapse formation is required for the development of neuronal structure in the functioning brain.

In the hippocampus, the mossy fiber pathway consists of unmyelinated axons projecting from granule cells in the dentate gyrus that terminate on modulatory hilar mossy cells and in Cornu Ammonis area 3 (CA3), a region involved in encoding short-term memory. These axons were first described as mossy fibers by Santiago Ramón y Cajal as they displayed varicosities along their lengths that gave them a mossy appearance. The axons that make up the pathway emerge from the basal portions of the granule cells and pass through the hilus of the dentate gyrus before entering the stratum lucidum of CA3. Granule cell synapses tend to be glutamatergic, though immunohistological data has indicated that some synapses contain neuropeptidergic elements including opiate peptides such as dynorphin and enkephalin. There is also evidence for co-localization of both GABAergic and glutamatergic neurotransmitters within mossy fiber terminals. GABAergic and glutamatergic co-localization in mossy fiber boutons has been observed primarily in the developing hippocampus, but in adulthood, evidence suggests that mossy fiber synapses may alternate which neurotransmitter is released through activity-dependent regulation.

Mossy fibers are one of the major inputs to cerebellum. There are many sources of this pathway, the largest of which is the cerebral cortex, which sends input to the cerebellum via the pontocerebellar pathway. Other contributors include the vestibular nerve and nuclei, the spinal cord, the reticular formation, and feedback from deep cerebellar nuclei. Axons enter the cerebellum via the middle and inferior cerebellar peduncles, where some branch to make contact with deep cerebellar nuclei. They ascend into the white matter of the cerebellum, where each axon branches to innervate granule cells in several cerebellar folia.

The anatomy of the cerebellum can be viewed at three levels. At the level of gross anatomy, the cerebellum consists of a tightly folded and crumpled layer of cortex, with white matter underneath, several deep nuclei embedded in the white matter, and a fluid-filled ventricle in the middle. At the intermediate level, the cerebellum and its auxiliary structures can be broken down into several hundred or thousand independently functioning modules or compartments known as microzones. At the microscopic level, each module consists of the same small set of neuronal elements, laid out with a highly stereotyped geometry.
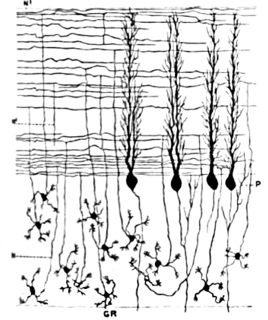
Cerebellar granule cells form the thick granular layer of the cerebellar cortex and are among the smallest neurons in the brain. Cerebellar granule cells are also the most numerous neurons in the brain: in humans, estimates of their total number average around 50 billion, which means that they constitute about 3/4 of the brain's neurons.

The name granule cell has been used for a number of different types of neuron whose only common feature is that they all have very small cell bodies. Granule cells are found within the granular layer of the cerebellum, the dentate gyrus of the hippocampus, the superficial layer of the dorsal cochlear nucleus, the olfactory bulb, and the cerebral cortex.

The rhombic lip is a posterior section of the developing metencephalon which can be recognized transiently within the vertebrate embryo. It extends posteriorly from the roof of the fourth ventricle to dorsal neuroepithelial cells. The rhombic lip can be divided into eight structural units based on rhombomeres 1-8 (r1-r8), which can be recognized at early stages of hindbrain development. Producing granule cells and five brainstem nuclei, the rhombic lip plays an important role in developing a complex cerebellar neural system.
Cartwheel cells are neurons of the dorsal cochlear nucleus (DCN) where they greatly outnumber the other inhibitory interneurons of the DCN. Their somas lie on the superficial side of the pyramidal layer of the DCN, and their dendrites receive input from the parallel fibres of the granule cell layer. Their axons do not extend beyond the dorsal cochlear nucleus but synapse with other cartwheel cells and pyramidal cells within the DCN releasing GABA and glycine onto their targets.

The cerebellar glomerulus is a small, intertwined mass of nerve fiber terminals in the granular layer of the cerebellar cortex. It consists of post-synaptic granule cell dendrites and pre-synaptic Golgi cell axon terminals surrounding the pre-synaptic terminals of mossy fibers.
Dendrodendritic synapses are connections between the dendrites of two different neurons. This is in contrast to the more common axodendritic synapse (chemical synapse) where the axon sends signals and the dendrite receives them. Dendrodendritic synapses are activated in a similar fashion to axodendritic synapses in respects to using a chemical synapse. These chemical synapses receive a depolarizing signal from an incoming action potential which results in an influx of calcium ions that permit release of Neurotransmitters to propagate the signal the post synaptic cell. There is also evidence of bi-directionality in signaling at dendrodendritic synapses. Ordinarily, one of the dendrites will display inhibitory effects while the other will display excitatory effects. The actual signaling mechanism utilizes Na+ and Ca2+ pumps in a similar manner to those found in axodendritic synapses.
An axo-axonic synapse is a type of synapse, formed by one neuron projecting its axon terminals onto another neuron’s axon.











