
Non-coding DNA (ncDNA) sequences are components of an organism's DNA that do not encode protein sequences. Some non-coding DNA is transcribed into functional non-coding RNA molecules. Other functional regions of the non-coding DNA fraction include regulatory sequences that control gene expression; scaffold attachment regions; origins of DNA replication; centromeres; and telomeres. Some non-coding regions appear to be mostly nonfunctional such as introns, pseudogenes, intergenic DNA, and fragments of transposons and viruses.

A non-coding RNA (ncRNA) is a functional RNA molecule that is not translated into a protein. The DNA sequence from which a functional non-coding RNA is transcribed is often called an RNA gene. Abundant and functionally important types of non-coding RNAs include transfer RNAs (tRNAs) and ribosomal RNAs (rRNAs), as well as small RNAs such as microRNAs, siRNAs, piRNAs, snoRNAs, snRNAs, exRNAs, scaRNAs and the long ncRNAs such as Xist and HOTAIR.

In biology, the word gene can have several different meanings. The Mendelian gene is a basic unit of heredity and the molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protein-coding genes and noncoding genes.

Interleukin enhancer-binding factor 3 is a protein that in humans is encoded by the ILF3 gene.
The perinucleolar compartment (PNC) is a subnuclear body characterized by its location at the periphery of the nucleolus. The PNC participates in the patterned compartmentalization inside the nucleus to organize the specialized functions. It is almost exclusively found in oncogenic cells and enriched with RNA binding proteins as well as RNA polymerase III transcripts.

Sjögren syndrome type B antigen (SS-B) also known as Lupus La protein is a protein that in humans is encoded by the SSB gene.

Tripartite motif-containing protein 21, also known as E3 ubiquitin-protein ligase TRIM21, is a protein that in humans is encoded by the TRIM21 gene. Alternatively spliced transcript variants for this gene have been described but the full-length nature of only one has been determined. It is expressed in most human tissues.

Chromatin assembly factor 1 subunit B is a protein that in humans is encoded by the CHAF1B gene.

Interleukin enhancer-binding factor 2 is a protein that in humans is encoded by the ILF2 gene.

60 kDa SS-A/Ro ribonucleoprotein is a protein that in humans is encoded by the TROVE2 gene.

Exosome component 4, also known as EXOSC4, is a human gene, which is part of the exosome complex.
3'-5' exoribonuclease CSL4 homolog is an enzyme that in humans is encoded by the EXOSC1 gene.

REX2, RNA exonuclease 2 homolog , also known as REXO2, is an enzyme which in humans is encoded by the REXO2 gene.

Zinc finger protein 330 is a protein that in humans is encoded by the ZNF330 gene.

Long non-coding RNAs are a type of RNA, generally defined as transcripts more than 200 nucleotides that are not translated into protein. This arbitrary limit distinguishes long ncRNAs from small non-coding RNAs, such as microRNAs (miRNAs), small interfering RNAs (siRNAs), Piwi-interacting RNAs (piRNAs), small nucleolar RNAs (snoRNAs), and other short RNAs. Long intervening/intergenic noncoding RNAs (lincRNAs) are sequences of lncRNA which do not overlap protein-coding genes.

HOTAIR is a human gene located between HOXC11 and HOXC12 on chromosome 12. It is the first example of an RNA expressed on one chromosome that has been found to influence transcription of HOXD cluster posterior genes located on chromosome 2. The sequence and function of HOTAIR is different in human and mouse. Sequence analysis of HOTAIR revealed that it exists in mammals, has poorly conserved sequences and considerably conserved structures, and has evolved faster than nearby HoxC genes. A subsequent study identified HOTAIR has 32 nucleotide long conserved noncoding element (CNE) that has a paralogous copy in HOXD cluster region, suggesting that the HOTAIR conserved sequences predates whole genome duplication events at the root of vertebrate. While the conserved sequence paralogous with HOXD cluster is 32 nucleotide long, the HOTAIR sequence conserved from human to fish is about 200 nucleotide long and is marked by active enhancer features.
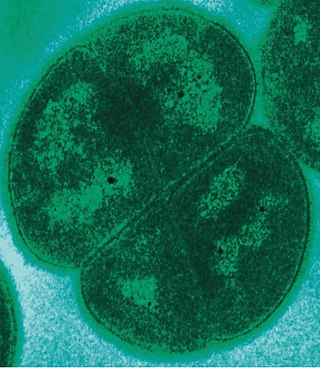
Deinococcus radiodurans is an extremophilic bacterium and one of the most radiation-resistant organisms known. It can survive cold, dehydration, vacuum, and acid, and therefore is known as a polyextremophile. It has been listed as the world's toughest known bacterium in The Guinness Book Of World Records.

sbRNA is a family of non-coding RNA first discovered in Caenorhabditis elegans. It was identified during a full transcriptome screen of the C. elegans cDNA library. Subsequent experimentation characterised sbRNA as having conserved 5' and 3' internal motifs which form a long paired stem which is interrupted with a bulge.
Deinococcus deserti is a Gram-negative, rod-shaped bacterium that belongs to the Deinococcaceae, a group of extremely radiotolerant bacteria. D. deserti and other Deinococcaceae exhibit an extraordinary ability to withstand ionizing radiation.

Sandra Lynn Wolin is an American microbiologist and physician-scientist specialized in biogenesis, function, and turnover of non-coding RNA. She is chief of the RNA Biology Laboratory at the National Cancer Institute.



















