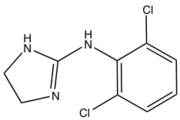
Imidazole is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-adjacent nitrogen atoms in meta-substitution.
Isoxazole is an electron-rich azole with an oxygen atom next to the nitrogen. It is also the class of compounds containing this ring. Isoxazolyl is the univalent radical derived from isoxazole.

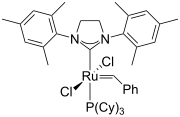
Alkyne metathesis is an organic reaction that entails the redistribution of alkyne chemical bonds. The reaction requires metal catalysts. Mechanistic studies show that the conversion proceeds via the intermediacy of metal alkylidyne complexes. The reaction is related to olefin metathesis.
Pyrazole is an organic compound with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazole is a weak base, with pKb 11.5 (pKa of the conjugated acid 2.49 at 25 °C). Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol.
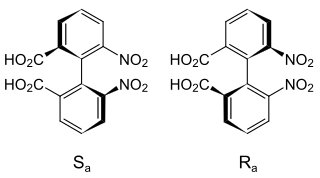
Atropisomers are stereoisomers arising because of hindered rotation about a single bond, where energy differences due to steric strain or other contributors create a barrier to rotation that is high enough to allow for isolation of individual conformers.
Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately 500,000 tonnes produced in 1998. Ethylenediamine is the first member of the so-called polyethylene amines.
The Letts nitrile synthesis is a chemical reaction of aromatic carboxylic acids with metal thiocyanates to form nitriles. The reaction includes the loss of carbon dioxide and potassium hydrosulfide. The polar basic substitution reaction was discovered in 1872 by Edmund A. Letts.

(+)-CPCA is a stimulant drug similar in structure to pethidine and to RTI-31, but nocaine is lacking the two-carbon bridge of RTI-31's tropane skeleton. This compound was first developed as a substitute agent for cocaine.

Troparil is a stimulant drug used in scientific research. Troparil is a phenyltropane-based dopamine reuptake inhibitor (DRI) that is derived from methylecgonidine. Troparil is a few times more potent than cocaine as a dopamine reuptake inhibitor, but is less potent as a serotonin reuptake inhibitor, and has a duration spanning a few times longer, since the phenyl ring is directly connected to the tropane ring through a non-hydrolyzable carbon-carbon bond. The lack of an ester linkage removes the local anesthetic action from the drug, so troparil is a pure stimulant. This change in activity also makes troparil slightly less cardiotoxic than cocaine. The most commonly used form of troparil is the tartrate salt, but the hydrochloride and naphthalenedisulfonate salts are also available, as well as the free base.

Carboxypeptidase A usually refers to the pancreatic exopeptidase that hydrolyzes peptide bonds of C-terminal residues with aromatic or aliphatic side-chains. Most scientists in the field now refer to this enzyme as CPA1, and to a related pancreatic carboxypeptidase as CPA2.
Imidazoline receptors are the primary receptors on which clonidine and other imidazolines act. There are three main classes of imidazoline receptor: I1 is involved in inhibition of the sympathetic nervous system to lower blood pressure, I2 has as yet uncertain functions but is implicated in several psychiatric conditions, and I3 regulates insulin secretion.

4-NEMD is a potent sedative drug which acts as a selective alpha-2 adrenergic agonist. It is closely related to dexmedetomidine but is several times more potent. Like other alpha-2 agonists, it produces sedative and muscle relaxant effects but without producing respiratory depression. It is not currently used in medicine but has been researched as the basis for a potential new generation of alpha-2 agonist drugs, which may have selectivity for the different subtypes of the alpha-2 receptor. It has two isomers, with the (S) isomer being the more potent, as with medetomidine. 4-NEMD was also investigated by the United States military as an anaesthetic agent, most likely for use in surgery but possibly also for use as a non-lethal incapacitating agent, although this has not been officially confirmed.
Dipeptidyl peptidase-4 inhibitors are enzyme inhibitors that inhibit the enzyme dipeptidyl peptidase-4 (DPP-4). They are used in the treatment of type 2 diabetes mellitus. Inhibition of the DPP-4 enzyme prolongs and enhances the activity of incretins that play an important role in insulin secretion and blood glucose control regulation. Type 2 diabetes mellitus is a chronic metabolic disease that results from inability of the β-cells in the pancreas to secrete sufficient amounts of insulin to meet the body's needs. Insulin resistance and increased hepatic glucose production can also play a role by increasing the body's demand for insulin. Current treatments, other than insulin supplementation, are sometimes not sufficient to achieve control and may cause undesirable side effects, such as weight gain and hypoglycemia. In recent years, new drugs have been developed, based on continuing research into the mechanism of insulin production and regulation of the metabolism of sugar in the body. The enzyme DPP-4 has been found to play a significant role.

Nutlins are cis-imidazoline analogs which inhibit the interaction between mdm2 and tumor suppressor p53, and which were discovered by screening a chemical library by Vassilev et al. Nutlin-1, nutlin-2, and nutlin-3 were all identified in the same screen; however, Nutlin-3 is the compound most commonly used in anti-cancer studies. Nutlin small molecules occupy p53 binding pocket of MDM2 and effectively disrupt the p53–MDM2 interaction that leads to activation of the p53 pathway in p53 wild-type cells. Inhibiting the interaction between mdm2 and p53 stabilizes p53, and is thought to selectively induce a growth-inhibiting state called senescence in cancer cells. These compounds are therefore thought to work best on tumors that contain normal or "wild-type" p53. Nutlin-3 has been shown to affect the production of p53 within minutes.

Oxazoline is a five-membered heterocyclic chemical compound containing one atom each of oxygen and nitrogen. It was likely first synthesized in 1884 but it was not until 5 years later that Siegmund Gabriel correctly assigned the structure. It was named in-line with the Hantzsch–Widman nomenclature and is part of a family of heterocyclic compounds, where it exists between oxazole and oxazolidine in terms of saturation.

JNJ-7925476 is a triple reuptake inhibitor antidepressant discovered by Johnson & Johnson, but never marketed.

Imidazothiazoles are a class of chemical compounds containing a bicyclic heterocycle consisting of an imidazole ring fused to a thiazole ring. The structure contains three non-carbon or heteroatoms: two nitrogen atoms and one sulfur atom. Imidazothiazole derivatives show a broad spectrum of in vitro, i.e. "in the petri dish", activity such as anticancer, antipsychotic, antimicrobial, antifungal, and anthelmintic.
Aminophosphonates are organophosphorus compounds with the formula (RO)2P(O)CR'2NR"2. These compounds are structural analogues of amino acids in which a carboxylic moiety is replaced by phosphonic acid or related groups. Acting as antagonists of amino acids, they inhibit enzymes involved in amino acid metabolism and thus affect the physiological activity of the cell. These effects may be exerted as antibacterial, plant growth regulatory or neuromodulatory. They can act as ligands, and heavy metal complexes with aminophosphonates have had medical applications investigated.
Shaomeng Wang is a Chinese-American chemist currently the Warner-Lambert/Parke-Davis Professor in Medicine at University of Michigan and a former Co-Editor-in-Chief at American Chemical Society's Journal of Medicinal Chemistry. A cited expert in his field, his interests are synthesis and design of moleculars, neurological diseases and computational and informatics. He was Elected as Fellow at the National Academy of Inventors in 2014. Dr. Wang was named to the AAAS Fellows Section on Pharmaceutical Sciences in 2019, and is the recipient of the Division of Medicinal Chemistry Award 2020 American Chemical Society.
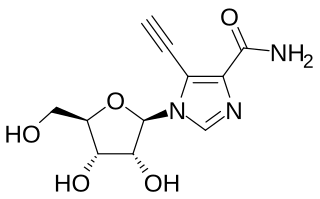
EICAR is a drug which acts as an inhibitor of the enzyme IMP dehydrogenase. It is a nucleoside derivative which has both anti-cancer and antiviral effects, and was originally developed for the treatment of leukemia, but was unsuccessful in human clinical trials. It has broad spectrum antiviral effects with activity against pox viruses, Semliki forest virus, Junin virus, reovirus, influenza, measles virus and respiratory syncytial virus among others, although it is not active against coronaviridae such as SARS-CoV-1. This useful spectrum of activity means that EICAR and related derivatives continue to be investigated for the treatment of viral diseases.