Related Research Articles

The adrenergic receptors or adrenoceptors are a class of G protein-coupled receptors that are targets of many catecholamines like norepinephrine (noradrenaline) and epinephrine (adrenaline) produced by the body, but also many medications like beta blockers, beta-2 (β2) agonists and alpha-2 (α2) agonists, which are used to treat high blood pressure and asthma, for example.

The N-methyl-D-aspartatereceptor (also known as the NMDA receptor or NMDAR), is a glutamate receptor and ion channel found in neurons. The NMDA receptor is one of three types of ionotropic glutamate receptors, the other two being AMPA and kainate receptors. Depending on its subunit composition, its ligands are glutamate and glycine (or D-serine). However, the binding of the ligands is typically not sufficient to open the channel as it may be blocked by Mg2+ ions which are only removed when the neuron is sufficiently depolarized. Thus, the channel acts as a “coincidence detector” and only once both of these conditions are met, the channel opens and it allows positively charged ions (cations) to flow through the cell membrane. The NMDA receptor is thought to be very important for controlling synaptic plasticity and mediating learning and memory functions.
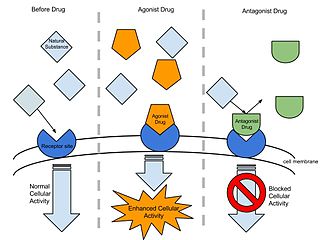
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist.

Clonidine, sold under the brand name Catapres among others, is an α2-adrenergic agonist medication used to treat high blood pressure, ADHD, drug withdrawal, menopausal flushing, diarrhea, spasticity, and certain pain conditions. It is used orally, by injection, or as a transdermal skin patch. Onset of action is typically within an hour with the effects on blood pressure lasting for up to eight hours.

A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins. They are sometimes called blockers; examples include alpha blockers, beta blockers, and calcium channel blockers. In pharmacology, antagonists have affinity but no efficacy for their cognate receptors, and binding will disrupt the interaction and inhibit the function of an agonist or inverse agonist at receptors. Antagonists mediate their effects by binding to the active site or to the allosteric site on a receptor, or they may interact at unique binding sites not normally involved in the biological regulation of the receptor's activity. Antagonist activity may be reversible or irreversible depending on the longevity of the antagonist–receptor complex, which, in turn, depends on the nature of antagonist–receptor binding. The majority of drug antagonists achieve their potency by competing with endogenous ligands or substrates at structurally defined binding sites on receptors.
Agmatine, also known as 4-aminobutyl-guanidine, was discovered in 1910 by Albrecht Kossel. It is a chemical substance which is naturally created from the amino acid arginine. Agmatine has been shown to exert modulatory action at multiple molecular targets, notably: neurotransmitter systems, ion channels, nitric oxide (NO) synthesis and polyamine metabolism and this provides bases for further research into potential applications.

Ligand-gated ion channels (LICs, LGIC), also commonly referred to as ionotropic receptors, are a group of transmembrane ion-channel proteins which open to allow ions such as Na+, K+, Ca2+, and/or Cl− to pass through the membrane in response to the binding of a chemical messenger (i.e. a ligand), such as a neurotransmitter.

The GABAA receptor (GABAAR) is an ionotropic receptor and ligand-gated ion channel. Its endogenous ligand is γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system. Upon opening, the GABAA receptor on the postsynaptic cell is selectively permeable to chloride ions (Cl−) and, to a lesser extent, bicarbonate ions (HCO3−). Depending on the membrane potential and the ionic concentration difference, this can result in ionic fluxes across the pore. If the membrane potential is higher than the equilibrium potential (also known as the reversal potential) for chloride ions, when the receptor is activated Cl− will flow into the cell. This causes an inhibitory effect on neurotransmission by diminishing the chance of a successful action potential occurring at the postsynaptic cell. The reversal potential of the GABAA-mediated inhibitory postsynaptic potential (IPSP) in normal solution is −70 mV, contrasting the GABAB IPSP (-100 mV).
The alpha-2 (α2) adrenergic receptor is a G protein-coupled receptor (GPCR) associated with the Gi heterotrimeric G-protein. It consists of three highly homologous subtypes, including α2A-, α2B-, and α2C-adrenergic. Some species other than humans express a fourth α2D-adrenergic receptor as well. Catecholamines like norepinephrine (noradrenaline) and epinephrine (adrenaline) signal through the α2-adrenergic receptor in the central and peripheral nervous systems.

NMDA receptor antagonists are a class of drugs that work to antagonize, or inhibit the action of, the N-Methyl-D-aspartate receptor (NMDAR). They are commonly used as anesthetics for animals and humans; the state of anesthesia they induce is referred to as dissociative anesthesia.

Alpha-adrenergic agonists are a class of sympathomimetic agents that selectively stimulates alpha adrenergic receptors. The alpha-adrenergic receptor has two subclasses α1 and α2. Alpha 2 receptors are associated with sympatholytic properties. Alpha-adrenergic agonists have the opposite function of alpha blockers. Alpha adrenoreceptor ligands mimic the action of epinephrine and norepinephrine signaling in the heart, smooth muscle and central nervous system, with norepinephrine being the highest affinity. The activation of α1 stimulates the membrane bound enzyme phospholipase C, and activation of α2 inhibits the enzyme adenylate cyclase. Inactivation of adenylate cyclase in turn leads to the inactivation of the secondary messenger cyclic adenosine monophosphate and induces smooth muscle and blood vessel constriction.

Cirazoline is a full agonist at the α1A adrenergic receptor, a partial agonist at both the α1B and α1D adrenergic receptors, and a nonselective antagonist to the α2 adrenergic receptor. It is believed that this combination of properties could make cirazoline an effective vasoconstricting agent.

An adrenergic antagonist is a drug that inhibits the function of adrenergic receptors. There are five adrenergic receptors, which are divided into two groups. The first group of receptors are the beta (β) adrenergic receptors. There are β1, β2, and β3 receptors. The second group contains the alpha (α) adrenoreceptors. There are only α1 and α2 receptors. Adrenergic receptors are located near the heart, kidneys, lungs, and gastrointestinal tract. There are also α-adreno receptors that are located on vascular smooth muscle.
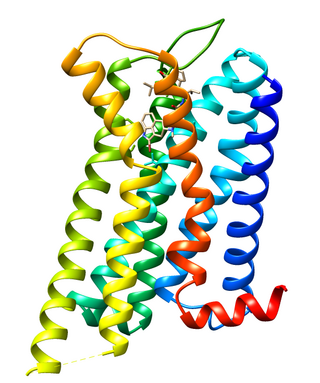
Dopamine receptor D2, also known as D2R, is a protein that, in humans, is encoded by the DRD2 gene. After work from Paul Greengard's lab had suggested that dopamine receptors were the site of action of antipsychotic drugs, several groups, including those of Solomon Snyder and Philip Seeman used a radiolabeled antipsychotic drug to identify what is now known as the dopamine D2 receptor. The dopamine D2 receptor is the main receptor for most antipsychotic drugs. The structure of DRD2 in complex with the atypical antipsychotic risperidone has been determined.

Idazoxan (INN) is a drug which is used in scientific research. It acts as both a selective α2 adrenergic receptor antagonist, and an antagonist for the imidazoline receptor. Idazoxan has been under investigation as an antidepressant, but it did not reach the market as such. More recently, it is under investigation as an adjunctive treatment in schizophrenia. Due to its alpha-2 receptor antagonism it is capable of enhancing therapeutic effects of antipsychotics, possibly by enhancing dopamine neurotransmission in the prefrontal cortex of the brain, a brain area thought to be involved in the pathogenesis of schizophrenia.
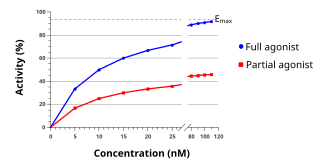
In pharmacology the term agonist-antagonist or mixed agonist/antagonist is used to refer to a drug which under some conditions behaves as an agonist while under other conditions, behaves as an antagonist.

Alazocine, also known more commonly as N-allylnormetazocine (NANM), is a synthetic opioid analgesic of the benzomorphan family related to metazocine, which was never marketed. In addition to its opioid activity, the drug is a sigma receptor agonist, and has been used widely in scientific research in studies of this receptor. Alazocine is described as a potent analgesic, psychotomimetic or hallucinogen, and opioid antagonist. Moreover, one of its enantiomers was the first compound that was found to selectively label the σ1 receptor, and led to the discovery and characterization of the receptor.
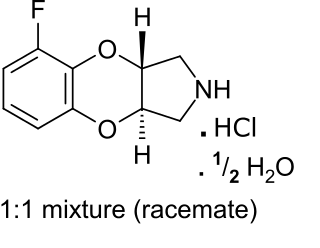
Fluparoxan is a potent α2-adrenergic receptor antagonist with excellent selectivity for this receptor over the α1-adrenergic receptor (2,630-fold), and is the only well-studied α2-adrenergic receptor antagonist in its structural family which does not antagonize any variant of the imidazoline receptor. It was shown to possess central α2-adrenoceptor antagonist activity after oral doses in man and was patented as an antidepressant by Glaxo in the early 1980s, but its development was discontinued when the compound failed to show a clear clinical advantage over existing therapies.

Tiamenidine (BAN, USAN, INN, also known as thiamenidine, Hoe 440) is an imidazoline compound that shares many of the pharmacological properties of clonidine. It is a centrally-acting α2 adrenergic receptor agonist (IC50 = 9.1 nM). It also acts as an α1-adrenergic receptor agonist to a far lesser extent (IC50 = 4.85 μM). In hypertensive volunteers, like clonidine, it significantly increased sinus node recovery time and lowered cardiac output. It was marketed (as tiamenidine hydrochloride) by Sanofi-Aventis under the brand name Sundralen for the management of essential hypertension.

Aganodine is a guanidine that activates presynaptic imidazoline receptors. Through its agonism at imidazoline receptors, aganodine inhibits the presynaptic release of norepinephrine.
References
- ↑ Head GA, Mayorov DN (January 2006). "Imidazoline receptors, novel agents and therapeutic potential". Cardiovasc Hematol Agents Med Chem. 4 (1): 17–32. doi:10.2174/187152506775268758. PMID 16529547. Archived from the original on April 14, 2013.
- ↑ Hamilton CA, Yakubu MA, Howie CA, Reid JL (1992). "Do Centrally-Acting Antihypertensive Drugs Act at Non-Adrenergic as well as Alpha-2 Adrenoceptor Sites?". Clinical and Experimental Hypertension Part A Theory and Practice. 14 (5): 815–835. doi:10.3109/10641969209036221. PMID 1327589.
- ↑ Hamilton CA, Yakubu MA, Jardine E, Reid JL (1991). "Imidazole binding sites in rabbit kidney and forebrain membranes". J Auton Pharmacol. 11 (5): 277–83. doi:10.1111/j.1474-8673.1991.tb00325.x. PMID 1939285.
- ↑ Regunathan, S; Reis, D J (April 1996). "Imidazoline Receptors and Their Endogenous Ligands". Annual Review of Pharmacology and Toxicology. 36 (1): 511–544. doi:10.1146/annurev.pa.36.040196.002455. PMID 8725400.
- ↑ Kawamura, Kazunori; Shimoda, Yoko; Kumata, Katsushi; Fujinaga, Masayuki; Yui, Joji; Yamasaki, Tomoteru; Xie, Lin; Hatori, Akiko; Wakizaka, Hidekatsu; Kurihara, Yusuke; Ogawa, Masanao; Nengaki, Nobuki; Zhang, Ming-Rong (April 2015). "In vivo evaluation of a new 18F-labeled PET ligand, [18F]FEBU, for the imaging of I2-imidazoline receptors". Nuclear Medicine and Biology. 42 (4): 406–412. doi:10.1016/j.nucmedbio.2014.12.014. PMID 25583220.
- ↑ GARCIA-SEVILLA, JESUS A.; ESCRIBA, PABLO V.; GUIMON, JOSE (June 1999). "Imidazoline Receptors and Human Brain Disorders". Annals of the New York Academy of Sciences. 881 (1 IMIDAZOLINE R): 392–409. Bibcode:1999NYASA.881..392G. doi:10.1111/j.1749-6632.1999.tb09388.x. PMID 10415944. S2CID 10081479.
- ↑ Head, G.; Mayorov, D. (1 January 2006). "Imidazoline Receptors, Novel Agents and Therapeutic Potential". Cardiovascular & Hematological Agents in Medicinal Chemistry. 4 (1): 17–32. doi:10.2174/187152506775268758. PMID 16529547.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Ernsberger, P; Graves, ME; Graff, LM; Zakieh, N; Nguyen, P; Collins, LA; Westbrooks, KL; Johnson, GG (12 July 1995). "I1-imidazoline receptors. Definition, characterization, distribution, and transmembrane signaling". Annals of the New York Academy of Sciences. 763: 22–42. doi:10.1111/j.1749-6632.1995.tb32388.x. PMID 7677333. S2CID 85739305.
- 1 2 Ernsberger P (June 1999). "The I1-imidazoline receptor and its cellular signaling pathways". Ann. N. Y. Acad. Sci. 881 (1): 35–53. Bibcode:1999NYASA.881...35E. doi:10.1111/j.1749-6632.1999.tb09339.x. PMID 10415895. S2CID 30065467. Archived from the original on 2009-01-08.
- ↑ Bousquet, P (June 2000). "Identification and characterization of I1 imidazoline receptors: their role in blood pressure regulation". American Journal of Hypertension. 13 (6 Pt 2): 84S–88S. doi: 10.1016/S0895-7061(00)00223-5 . PMID 10921526.
- ↑ Bousquet, P (November 2001). "I1 receptors, cardiovascular function, and metabolism". American Journal of Hypertension. 14 (11 Pt 2): 317S–321S. doi: 10.1016/S0895-7061(01)02238-5 . PMID 11721890.
- 1 2 3 4 5 6 7 8 9 Li, JX (16 March 2017). "Imidazoline I2 receptors: An update". Pharmacology & Therapeutics. 178: 48–56. doi:10.1016/j.pharmthera.2017.03.009. PMC 5600648 . PMID 28322973.
- ↑ McDonald, GR; Olivieri, A; Ramsay, RR; Holt, A (December 2010). "On the formation and nature of the imidazoline I2 binding site on human monoamine oxidase-B". Pharmacological Research. 62 (6): 475–88. doi:10.1016/j.phrs.2010.09.001. PMID 20832472.
- ↑ Morgan, NG; Chan, SL (September 2001). "Imidazoline binding sites in the endocrine pancreas: can they fulfil their potential as targets for the development of new insulin secretagogues?". Current Pharmaceutical Design. 7 (14): 1413–31. doi:10.2174/1381612013397366. PMID 11472276.
- ↑ Sánchez-Blázquez, P; Boronat, MA; Olmos, G; García-Sevilla, JA; Garzón, J (May 2000). "Activation of I(2)-imidazoline receptors enhances supraspinal morphine analgesia in mice: a model to detect agonist and antagonist activities at these receptors". British Journal of Pharmacology. 130 (1): 146–52. doi:10.1038/sj.bjp.0703294. PMC 1572044 . PMID 10781010.
- 1 2 3 Qiu, Y; He, XH; Zhang, Y; Li, JX (13 October 2014). "Discriminative stimulus effects of the novel imidazoline I₂ receptor ligand CR4056 in rats". Scientific Reports. 4: 6605. Bibcode:2014NatSR...4E6605Q. doi:10.1038/srep06605. PMC 4194429 . PMID 25308382.
- ↑ Han, Z; et al. (2013). "Fast, non-competitive and reversible inhibition of NMDA-activated currents by 2-BFI confers neuroprotection". PLOS ONE. 8 (5): e64894. Bibcode:2013PLoSO...864894H. doi: 10.1371/journal.pone.0064894 . PMC 3669129 . PMID 23741413.
- ↑ Reis DJ, Piletz JE (November 1997). "The imidazoline receptor in control of blood pressure by clonidine and allied drugs" (PDF). Am. J. Physiol. 273 (5 Pt 2): R1569–71. doi:10.1152/ajpregu.1997.273.5.R1569. PMID 9374795.
- ↑ Bousquet P (2002). "I1 imidazoline receptors: From the pharmacological basis to the therapeutic application" (PDF). Journal für Hypertonie. 6 (4): 6–9.
- ↑ Ray, Thomas S. (2010-02-02). Manzoni, Olivier Jacques (ed.). "Psychedelics and the Human Receptorome". PLOS ONE. 5 (2): e9019. Bibcode:2010PLoSO...5.9019R. doi: 10.1371/journal.pone.0009019 . ISSN 1932-6203. PMC 2814854 . PMID 20126400.