
The photoelectric effect is the emission of electrons when electromagnetic radiation, such as light, hits a material. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physics, solid state, and quantum chemistry to draw inferences about the properties of atoms, molecules and solids. The effect has found use in electronic devices specialized for light detection and precisely timed electron emission.
Atomic, molecular, and optical physics (AMO) is the study of matter–matter and light–matter interactions, at the scale of one or a few atoms and energy scales around several electron volts. The three areas are closely interrelated. AMO theory includes classical, semi-classical and quantum treatments. Typically, the theory and applications of emission, absorption, scattering of electromagnetic radiation (light) from excited atoms and molecules, analysis of spectroscopy, generation of lasers and masers, and the optical properties of matter in general, fall into these categories.

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.
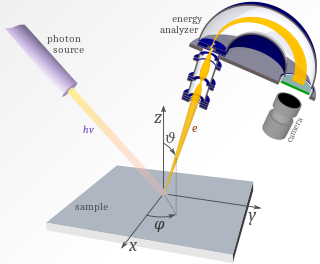
Photoemission spectroscopy (PES), also known as photoelectron spectroscopy, refers to energy measurement of electrons emitted from solids, gases or liquids by the photoelectric effect, in order to determine the binding energies of electrons in the substance. The term refers to various techniques, depending on whether the ionization energy is provided by X-ray, XUV or UV photons. Regardless of the incident photon beam, however, all photoelectron spectroscopy revolves around the general theme of surface analysis by measuring the ejected electrons.

Photoionization is the physical process in which an ion is formed from the interaction of a photon with an atom or molecule.
Tunnel ionization is a process in which electrons in an atom pass through the potential barrier and escape from the atom. In an intense electric field, the potential barrier of an atom (molecule) is distorted drastically. Therefore, as the length of the barrier that electrons have to pass decreases, the electrons can escape from the atom's potential more easily. Tunneling Ionization is a quantum mechanical phenomenon, since in the classical picture an electron does not have sufficient energy to overcome the potential barrier of the atom.

Attosecond physics, also known as attophysics, or more generally attosecond science, is a branch of physics that deals with light-matter interaction phenomena wherein attosecond photon pulses are used to unravel dynamical processes in matter with unprecedented time resolution.
Rydberg ionization spectroscopy is a spectroscopy technique in which multiple photons are absorbed by an atom causing the removal of an electron to form an ion.

Resonance-enhanced multiphoton ionization (REMPI) is a technique applied to the spectroscopy of atoms and small molecules. In practice, a tunable laser can be used to access an excited intermediate state. The selection rules associated with a two-photon or other multiphoton photoabsorption are different from the selection rules for a single photon transition. The REMPI technique typically involves a resonant single or multiple photon absorption to an electronically excited intermediate state followed by another photon which ionizes the atom or molecule. The light intensity to achieve a typical multiphoton transition is generally significantly larger than the light intensity to achieve a single photon photoabsorption. Because of this, a subsequent photoabsorption is often very likely. An ion and a free electron will result if the photons have imparted enough energy to exceed the ionization threshold energy of the system. In many cases, REMPI provides spectroscopic information that can be unavailable to single photon spectroscopic methods, for example rotational structure in molecules is easily seen with this technique.
X-ray absorption near edge structure (XANES), also known as near edge X-ray absorption fine structure (NEXAFS), is a type of absorption spectroscopy that indicates the features in the X-ray absorption spectra (XAS) of condensed matter due to the photoabsorption cross section for electronic transitions from an atomic core level to final states in the energy region of 50–100 eV above the selected atomic core level ionization energy, where the wavelength of the photoelectron is larger than the interatomic distance between the absorbing atom and its first neighbour atoms.
This page deals with the electron affinity as a property of isolated atoms or molecules. Solid state electron affinities are not listed here.
Ultraviolet photoelectron spectroscopy (UPS) refers to the measurement of kinetic energy spectra of photoelectrons emitted by molecules which have absorbed ultraviolet photons, in order to determine molecular orbital energies in the valence region.
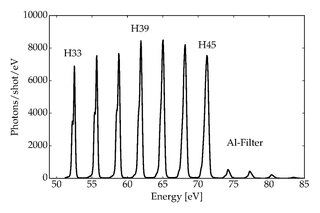
High-harmonic generation (HHG) is a non-linear process during which a target is illuminated by an intense laser pulse. Under such conditions, the sample will emit the high harmonics of the generation beam. Due to the coherent nature of the process, high-harmonics generation is a prerequisite of attosecond physics.
Photoelectrochemical processes are processes in photoelectrochemistry; they usually involve transforming light into other forms of energy. These processes apply to photochemistry, optically pumped lasers, sensitized solar cells, luminescence, and photochromism.
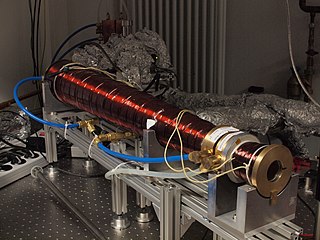
In atomic physics, a Zeeman slower is a scientific instrument that is commonly used in atomic physics to slow and cool a beam of hot atoms to speeds of several meters per second and temperatures below a kelvin. The gas-phase atoms used in atomic physics are often generated in an oven by heating a solid or liquid atomic sample to temperatures where the vapor pressure is high enough that a substantial number of atoms are in the gas phase. These atoms effuse out of a hole in the oven with average speeds on the order of hundreds of m/s and large velocity distributions. The Zeeman slower is attached close to where the hot atoms exit the oven and are used to slow them to less than 10 m/s (slowing) with a very small velocity spread (cooling).
Photoelectron photoion coincidence spectroscopy (PEPICO) is a combination of photoionization mass spectrometry and photoelectron spectroscopy. It is largely based on the photoelectric effect. Free molecules from a gas-phase sample are ionized by incident vacuum ultraviolet (VUV) radiation. In the ensuing photoionization, a cation and a photoelectron are formed for each sample molecule. The mass of the photoion is determined by time-of-flight mass spectrometry, whereas, in current setups, photoelectrons are typically detected by velocity map imaging. Electron times-of-flight are three orders of magnitude smaller than those of ions, which allows electron detection to be used as a time stamp for the ionization event, starting the clock for the ion time-of-flight analysis. In contrast with pulsed experiments, such as REMPI, in which the light pulse must act as the time stamp, this allows to use continuous light sources, e.g. a discharge lamp or a synchrotron light source. No more than several ion–electron pairs are present simultaneously in the instrument, and the electron–ion pairs belonging to a single photoionization event can be identified and detected in delayed coincidence.
Double ionization is a process of formation of doubly charged ions when laser radiation is exerted on neutral atoms or molecules. Double ionization is usually less probable than single-electron ionization. Two types of double ionization are distinguished: sequential and non-sequential.
Bond softening is an effect of reducing the strength of a chemical bond by strong laser fields. To make this effect significant, the strength of the electric field in the laser light has to be comparable with the electric field the bonding electron "feels" from the nuclei of the molecule. Such fields are typically in the range of 1–10 V/Å, which corresponds to laser intensities 1013–1015 W/cm2. Nowadays, these intensities are routinely achievable from table-top Ti:Sapphire lasers.
Bond hardening is a process of creating a new chemical bond by strong laser fields—an effect opposite to bond softening. However, it is not opposite in the sense that the bond becomes stronger, but in the sense that the molecule enters a state that is diametrically opposite to the bond-softened state. Such states require laser pulses of high intensity, in the range of 1013–1015 W/cm2, and they disappear once the pulse is gone.

The Collinear Resonance Ionization Spectroscopy (CRIS) experiment is located in the ISOLDE facility at CERN. The experiment aims to study ground-state properties of exotic nuclei and produce high purity isomeric beams used for decay studies. CRIS does this by using the high resolution technique of fast beam collinear laser spectroscopy, with the high efficiency technique of resonance ionization.













