Related Research Articles

Meconium aspiration syndrome (MAS) also known as neonatal aspiration of meconium is a medical condition affecting newborn infants. It describes the spectrum of disorders and pathophysiology of newborns born in meconium-stained amniotic fluid (MSAF) and have meconium within their lungs. Therefore, MAS has a wide range of severity depending on what conditions and complications develop after parturition. Furthermore, the pathophysiology of MAS is multifactorial and extremely complex which is why it is the leading cause of morbidity and mortality in term infants.
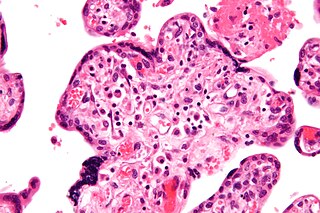
Intrauterine growth restriction (IUGR), or fetal growth restriction, is the poor growth of a fetus while in the womb during pregnancy. IUGR is defined by clinical features of malnutrition and evidence of reduced growth regardless of an infant's birth weight percentile. The causes of IUGR are broad and may involve maternal, fetal, or placental complications.

Dexamethasone is a glucocorticoid medication used to treat rheumatic problems, a number of skin diseases, severe allergies, asthma, chronic obstructive lung disease, croup, brain swelling, eye pain following eye surgery, superior vena cava syndrome, and along with antibiotics in tuberculosis. In adrenocortical insufficiency, it may be used in combination with a mineralocorticoid medication such as fludrocortisone. In preterm labor, it may be used to improve outcomes in the baby. It may be given by mouth, as an injection into a muscle, as an injection into a vein, as a topical cream or ointment for the skin or as a topical ophthalmic solution to the eye. The effects of dexamethasone are frequently seen within a day and last for about three days.

Preterm birth, also known as premature birth, is the birth of a baby at fewer than 37 weeks gestational age, as opposed to full-term delivery at approximately 40 weeks. Extreme preterm is less than 28 weeks, very early preterm birth is between 28 and 32 weeks, early preterm birth occurs between 32 and 34 weeks, late preterm birth is between 34 and 36 weeks' gestation. These babies are also known as premature babies or colloquially preemies or premmies. Symptoms of preterm labor include uterine contractions which occur more often than every ten minutes and/or the leaking of fluid from the vagina before 37 weeks. Premature infants are at greater risk for cerebral palsy, delays in development, hearing problems and problems with their vision. The earlier a baby is born, the greater these risks will be.
Fetal distress, also known as non-reassuring fetal status, is a condition during pregnancy or labor in which the fetus shows signs of inadequate oxygenation. Due to its imprecision, the term "fetal distress" has fallen out of use in American obstetrics. The term "non-reassuring fetal status" has largely replaced it. It is characterized by changes in fetal movement, growth, heart rate, and presence of meconium stained fluid.

Infant respiratory distress syndrome (IRDS), also called respiratory distress syndrome of newborn, or increasingly surfactant deficiency disorder (SDD), and previously called hyaline membrane disease (HMD), is a syndrome in premature infants caused by developmental insufficiency of pulmonary surfactant production and structural immaturity in the lungs. It can also be a consequence of neonatal infection and can result from a genetic problem with the production of surfactant-associated proteins.
Transient tachypnea of the newborn is a respiratory problem that can be seen in the newborn shortly after delivery. It is caused by retained fetal lung fluid due to impaired clearance mechanisms. It is the most common cause of respiratory distress in term neonates. It consists of a period of tachypnea (rapid breathing. Usually, this condition resolves over 24–72 hours. Treatment is supportive and may include supplemental oxygen and antibiotics. The chest x-ray shows hyperinflation of the lungs including prominent pulmonary vascular markings, flattening of the diaphragm, and fluid in the horizontal fissure of the right lung.
Tocolytics are medications used to suppress premature labor. Preterm birth accounts for 70% of neonatal deaths. Therefore, tocolytic therapy is provided when delivery would result in premature birth, postponing delivery long enough for the administration of glucocorticoids, which accelerate fetal lung maturity but may require one to two days to take effect.
Fetal viability is the ability of a human fetus to survive outside the uterus. Medical viability is generally considered to be between 23 and 24 weeks gestational age. Viability depends upon factors such as birth weight, gestational age, and the availability of advanced medical care. In low-income countries, half of newborns born at or below 32 weeks gestational age died due to a lack of medical access; in high-income countries, the vast majority of newborns born above 24 weeks gestational age survive.

Betamethasone is a steroid medication. It is used for a number of diseases including rheumatic disorders such as rheumatoid arthritis and systemic lupus erythematosus, skin diseases such as dermatitis and psoriasis, allergic conditions such as asthma and angioedema, preterm labor to speed the development of the baby's lungs, Crohn's disease, cancers such as leukemia, and along with fludrocortisone for adrenocortical insufficiency, among others. It can be taken by mouth, injected into a muscle, or applied to the skin, typically in cream, lotion, or liquid forms.

Prelabor rupture of membranes (PROM), previously known as premature rupture of membranes, is breakage of the amniotic sac before the onset of labor. Women usually experience a painless gush or a steady leakage of fluid from the vagina. Complications in the baby may include premature birth, cord compression, and infection. Complications in the mother may include placental abruption and postpartum endometritis.

Bronchopulmonary dysplasia is a chronic lung disease which affects premature infants. Premature (preterm) infants who require treatment with supplemental oxygen or require long-term oxygen are at a higher risk. The alveoli that are present tend to not be mature enough to function normally. It is also more common in infants with low birth weight (LBW) and those who receive prolonged mechanical ventilation to treat respiratory distress syndrome. It results in significant morbidity and mortality. The definition of bronchopulmonary dysplasia has continued to evolve primarily due to changes in the population, such as more survivors at earlier gestational ages, and improved neonatal management including surfactant, antenatal glucocorticoid therapy, and less aggressive mechanical ventilation.
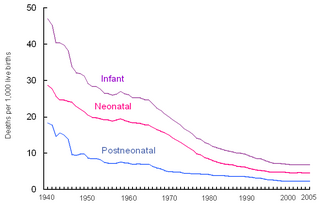
Perinatal mortality (PNM) is the death of a fetus or neonate and is the basis to calculate the perinatal mortality rate. Perinatal means "relating to the period starting a few weeks before birth and including the birth and a few weeks after birth."
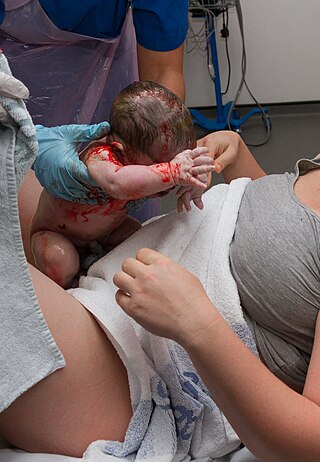
Maternal–fetal medicine (MFM), also known as perinatology, is a branch of medicine that focuses on managing health concerns of the mother and fetus prior to, during, and shortly after pregnancy.

Pulmonary hypoplasia is incomplete development of the lungs, resulting in an abnormally low number or small size of bronchopulmonary segments or alveoli. A congenital malformation, it most often occurs secondary to other fetal abnormalities that interfere with normal development of the lungs. Primary (idiopathic) pulmonary hypoplasia is rare and usually not associated with other maternal or fetal abnormalities.

The lecithin–sphingomyelin ratio is a test of fetal amniotic fluid to assess for fetal lung immaturity. Lungs require surfactant, a soap-like substance, to lower the surface pressure of the alveoli in the lungs. This is especially important for premature babies trying to expand their lungs after birth. Surfactant is a mixture of lipids, proteins, and glycoproteins, lecithin and sphingomyelin being two of them. Lecithin makes the surfactant mixture more effective.
Surfactant therapy is the medical administration of exogenous surfactant. Surfactants used in this manner are typically instilled directly into the trachea. When a baby comes out of the womb and the lungs are not developed yet, they require administration of surfactant in order to process oxygen and survive. This condition that the baby has is called newborn respiratory distress syndrome, and it is treatable. Surfactant coat the smallest parts of the lungs called the alveoli and helps for oxygen to go in and for carbon dioxide to go out. How surfactant does this is by not allowing the alveoli to collapse and to retain their inflated shape when the baby exhales.

Henrik Verder is a pediatrician and the inventor of the INSURE and LISA methods combined with nasal CPAP. In 1989 he used this pioneering method to successfully treat the first premature infant with severe RDS. Verder is a significant researcher within the field of paediatrics, with more than 50 publications and over 500 citations.
Christian P. Speer is a German pediatrician and Professor of Pediatrics specialized in neonatology at the Julius Maximilian University of Würzburg. Speer is known for his scientific and educational contributions in neonatal medicine.

Henry Lewis Halliday was a British-Irish peaditrician and neonatologist. In 2021, Halliday was awarded the James Spence Medal for research into neonatology, for coordinating two of the largest neonatal multicentre trials for prevention and treatment of a number of neonatal respiratory illnesses and for a breakthrough in the development of a new lung surfactant that brought relief to very small babies suffering from infant respiratory distress syndrome (RDS).
References
- 1 2 3 4 5 6 7 McGoldrick E, Stewart F, Parker R, Dalziel SR (December 2020). "Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth". The Cochrane Database of Systematic Reviews (published December 25, 2020). 12 (2): CD004454. doi:10.1002/14651858.CD004454.pub4. PMC 8094626 . PMID 33368142.
- ↑ Liggins GC, Howie RN (October 1972). "A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants". Pediatrics. 50 (4): 515–525. doi:10.1542/peds.50.4.515. PMID 4561295. S2CID 37818386.
- ↑ Mwansa-Kambafwile J, Cousens S, Hansen T, Lawn JE (April 2010). "Antenatal steroids in preterm labour for the prevention of neonatal deaths due to complications of preterm birth". International Journal of Epidemiology. 39 (Supplement 1): i122–i133. doi:10.1093/ije/dyq029. PMC 2845868 . PMID 20348115.
- ↑ Abbasi S, Oxford C, Gerdes J, Sehdev H, Ludmir J (January 2010). "Antenatal corticosteroids prior to 24 weeks' gestation and neonatal outcome of extremely low birth weight infants". American Journal of Perinatology. 27 (1): 61–66. doi:10.1055/s-0029-1223269. PMID 19544249. S2CID 259999936.
- ↑ Ment LR, Oh W, Ehrenkranz RA, Philip AG, Duncan CC, Makuch RW (March 1995). "Antenatal steroids, delivery mode, and intraventricular hemorrhage in preterm infants". American Journal of Obstetrics and Gynecology. 172 (3): 795–800. doi:10.1016/0002-9378(95)90001-2. PMID 7892866.
- ↑ Oladapo OT, Vogel JP, Piaggio G, Nguyen MH, Althabe F, Gülmezoglu AM, et al. (December 2020). "Antenatal Dexamethasone for Early Preterm Birth in Low-Resource Countries". The New England Journal of Medicine. 383 (26): 2514–2525. doi:10.1056/NEJMoa2022398. PMC 7660991 . PMID 33095526.
- 1 2 3 4 "Antenatal Corticosteroid Therapy for Fetal Maturation". www.acog.org. Retrieved 2020-12-01.
- ↑ Vidaeff AC, Ramin SM (June 2011). "Antenatal corticosteroids after preterm premature rupture of membranes". Clinical Obstetrics and Gynecology. 54 (2): 337–343. doi:10.1097/GRF.0b013e318217d85b. PMID 21508704. S2CID 205616406.
- ↑ Harding JE, Pang J, Knight DB, Liggins GC (January 2001). "Do antenatal corticosteroids help in the setting of preterm rupture of membranes?". American Journal of Obstetrics and Gynecology. 184 (2): 131–139. doi:10.1067/mob.2001.108331. PMID 11174492.
- 1 2 3 Asztalos EV, Murphy KE, Matthews SG (October 2020). "A Growing Dilemma: Antenatal Corticosteroids and Long-Term Consequences". American Journal of Perinatology. 39 (6): s–0040–1718573. doi:10.1055/s-0040-1718573. PMID 33053595. S2CID 222421170.
- ↑ Ninan K, Liyanage SK, Murphy KE, Asztalos EV, McDonald SD (April 2022). "Evaluation of Long-term Outcomes Associated With Preterm Exposure to Antenatal Corticosteroids: A Systematic Review and Meta-analysis". JAMA Pediatrics. 176 (6): e220483. doi:10.1001/jamapediatrics.2022.0483. PMC 9002717 . PMID 35404395. S2CID 248083996.
- ↑ Quinlivan JA, Beazley LD, Evans SF, Newnham JP, Dunlop SA (February 2000). "Retinal maturation is delayed by repeated, but not single, maternal injections of betamethasone in sheep". Eye. 14 (1): 93–98. doi: 10.1038/eye.2000.20 . PMID 10755109. S2CID 45299374.
- ↑ Church MW, Adams BR, Anumba JI, Jackson DA, Kruger ML, Jen KL (2012-01-01). "Repeated antenatal corticosteroid treatments adversely affect neural transmission time and auditory thresholds in laboratory rats". Neurotoxicology and Teratology. 34 (1): 196–205. doi:10.1016/j.ntt.2011.09.004. PMC 3268869 . PMID 21963399.
- ↑ Asztalos E, Willan A, Murphy K, Matthews S, Ohlsson A, Saigal S, et al. (August 2014). "Association between gestational age at birth, antenatal corticosteroids, and outcomes at 5 years: multiple courses of antenatal corticosteroids for preterm birth study at 5 years of age (MACS-5)". BMC Pregnancy and Childbirth. 14 (1): 272. doi: 10.1186/1471-2393-14-272 . PMC 4261573 . PMID 25123162.
- ↑ Miracle X, Di Renzo GC, Stark A, Fanaroff A, Carbonell-Estrany X, Saling E (2008-01-01). "Guideline for the use of antenatal corticosteroids for fetal maturation". Journal of Perinatal Medicine. 36 (3): 191–196. doi:10.1515/JPM.2008.032. PMID 18576926.
- ↑ Engle WA (February 2008). "Surfactant-replacement therapy for respiratory distress in the preterm and term neonate". Pediatrics. 121 (2): 419–432. doi:10.1542/peds.2007-3283. PMID 18245434. S2CID 12550991.
- ↑ "Recommendations for Use of Antenatal Corticosteroids". Perinatology.com. Retrieved 6 January 2014.
- ↑ "Medscape Obstetrics".(subscription required)
- ↑ "UK National Health Service". Archived from the original on 2012-09-03.
- ↑ McEvoy C, Schilling D, Spitale P, Peters D, O'Malley J, Durand M (May 2008). "Decreased respiratory compliance in infants less than or equal to 32 weeks' gestation, delivered more than 7 days after antenatal steroid therapy". Pediatrics. 121 (5): e1032–e1038. doi:10.1542/peds.2007-2608. PMID 18450845. S2CID 11806218.
- ↑ Chow, SSW, Creighton, P, Chambers, GM, Lui, K. 2020. "Report of the Australian and New Zealand Neonatal Network" 2018. Sydney: ANZNN.
- ↑ "Antenatal corticosteroids given to women prior to birth to improve fetal, infant, child and adult health: Clinical Practice Guidelines" (PDF). Antenatal Corticosteroid Clinical Practice Guidelines Panel. 2015. Liggins Institute, The University of Auckland, Auckland. New Zealand.
- ↑ "Guideline for the Management of Hypertensive Disorders of Pregnancy" (PDF). Society of Obstetric Medicine of Australia and New Zealand. 2014.
- ↑ Skoll A, Boutin A, Bujold E, Burrows J, Crane J, Geary M, et al. (September 2018). "No. 364-Antenatal Corticosteroid Therapy for Improving Neonatal Outcomes". Journal of Obstetrics and Gynaecology Canada. 40 (9): 1219–1239. doi:10.1016/j.jogc.2018.04.018. PMID 30268316. S2CID 52893292.
- ↑ "Preterm labour and birth" (PDF). National Institute for Health and Care Excellence. 2015.
- ↑ "WHO | WHO recommendations on interventions to improve preterm birth outcomes". WHO. Retrieved 2020-12-01.
- ↑ "Dexamethasone versus betamethasone as an antenatal corticosteroid (ACS)" (PDF). UN Commission / Born Too Soon Care Antenatal Corticosteroids Working Group. August 20, 2013. Archived from the original (PDF) on 6 January 2014. Retrieved 6 January 2014.
- ↑ "Antenatal Steroid Video". Archived from the original on 2014-01-07. Retrieved 2011-12-27.
- ↑ Fanaroff AA, Hack M (October 1999). "Periventricular leukomalacia--prospects for prevention". The New England Journal of Medicine. 341 (16): 1229–1231. doi:10.1056/NEJM199910143411611. PMID 10519903.
- ↑ Williams MJ, Ramson JA, Brownfoot FC (2022-08-09). "Different corticosteroids and regimens for accelerating fetal lung maturation for babies at risk of preterm birth". The Cochrane Database of Systematic Reviews. 2022 (8): CD006764. doi:10.1002/14651858.CD006764.pub4. ISSN 1469-493X. PMC 9362990 . PMID 35943347.
- ↑ Lee BH, Stoll BJ, McDonald SA, Higgins RD (February 2008). "Neurodevelopmental outcomes of extremely low birth weight infants exposed prenatally to dexamethasone versus betamethasone". Pediatrics. 121 (2): 289–296. doi:10.1542/peds.2007-1103. PMID 18245420. S2CID 1550943.
- 1 2 Vyas J, Kotecha S (September 1997). "Effects of antenatal and postnatal corticosteroids on the preterm lung". Archives of Disease in Childhood. Fetal and Neonatal Edition. 77 (2): F147–F150. doi:10.1136/fn.77.2.f147. PMC 1720703 . PMID 9377142.
- ↑ Farrell PM (May 1977). "Fetal lung development and the influence of glucocorticoids on pulmonary surfactant". Journal of Steroid Biochemistry. 8 (5): 463–470. doi:10.1016/0022-4731(77)90248-5. PMID 579641.
- ↑ McMaster Children's Hospital (July 2018). "How surfactant helps your baby's lungs" (PDF). Hamilton Health Sciences. Retrieved 28 November 2020.
- ↑ Chakraborty M, Kotecha S (2013-12-01). "Pulmonary surfactant in newborn infants and children". Breathe. 9 (6): 476–488. doi: 10.1183/20734735.006513 . ISSN 1810-6838.
- ↑ Liggins GC (December 1969). "Premature delivery of foetal lambs infused with glucocorticoids". The Journal of Endocrinology. 45 (4): 515–523. doi:10.1677/joe.0.0450515. PMID 5366112.
- 1 2 3 4 Norwitz ER, Greenberg JA (2010). "Beyond antenatal corticosteroids: what did mont liggins teach us?". Reviews in Obstetrics & Gynecology. 3 (3): 79–80. PMC 3046760 . PMID 21364857.
- 1 2 3 4 Liggins GC, Howie RN (October 1972). "A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants". Pediatrics. 50 (4): 515–525. doi:10.1542/peds.50.4.515. PMID 4561295. S2CID 37818386.