The actinide or actinoid series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.
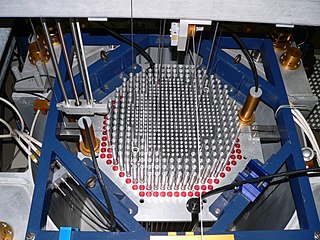
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from nuclear fission is passed to a working fluid, which in turn runs through steam turbines. These either drive a ship's propellers or turn electrical generators' shafts. Nuclear generated steam in principle can be used for industrial process heat or for district heating. Some reactors are used to produce isotopes for medical and industrial use, or for production of weapons-grade plutonium. As of 2022, the International Atomic Energy Agency reports there are 422 nuclear power reactors and 223 nuclear research reactors in operation around the world.

Uranium is a chemical element; it has symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium radioactively decays by emitting an alpha particle. The half-life of this decay varies between 159,200 and 4.5 billion years for different isotopes, making them useful for dating the age of the Earth. The most common isotopes in natural uranium are uranium-238 and uranium-235. Uranium has the highest atomic weight of the primordially occurring elements. Its density is about 70% higher than that of lead and slightly lower than that of gold or tungsten. It occurs naturally in low concentrations of a few parts per million in soil, rock and water, and is commercially extracted from uranium-bearing minerals such as uraninite.
Enriched uranium is a type of uranium in which the percent composition of uranium-235 has been increased through the process of isotope separation. Naturally-occurring uranium is composed of three major isotopes: uranium-238, uranium-235, and uranium-234. 235U is the only nuclide existing in nature that is fissile with thermal neutrons.

Uranium-235 is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exists in nature as a primordial nuclide.

Yellowcake is a type of uranium concentrate powder obtained from leach solutions, in an intermediate step in the processing of uranium ores. It is a step in the processing of uranium after it has been mined but before fuel fabrication or uranium enrichment. Yellowcake concentrates are prepared by various extraction and refining methods, depending on the types of ores. Typically, yellowcakes are obtained through the milling and chemical processing of uranium ore, forming a coarse powder that has a pungent odor, is insoluble in water, and contains about 80% uranium oxide, which melts at approximately 2880 °C.

A fast-neutron reactor (FNR) or fast-spectrum reactor or simply a fast reactor is a category of nuclear reactor in which the fission chain reaction is sustained by fast neutrons, as opposed to slow thermal neutrons used in thermal-neutron reactors. Such a fast reactor needs no neutron moderator, but requires fuel that is relatively rich in fissile material when compared to that required for a thermal-neutron reactor. Around 20 land based fast reactors have been built, accumulating over 400 reactor years of operation globally. The largest of this was the Superphénix Sodium cooled fast reactor in France that was designed to deliver 1,242 MWe. Fast reactors have been intensely studied since the 1950s, as they provide certain advantages over the existing fleet of water cooled and water moderated reactors. These are:

The light-water reactor (LWR) is a type of thermal-neutron reactor that uses normal water, as opposed to heavy water, as both its coolant and neutron moderator; furthermore a solid form of fissile elements is used as fuel. Thermal-neutron reactors are the most common type of nuclear reactor, and light-water reactors are the most common type of thermal-neutron reactor.

Uranium-234 is an isotope of uranium. In natural uranium and in uranium ore, 234U occurs as an indirect decay product of uranium-238, but it makes up only 0.0055% of the raw uranium because its half-life of just 245,500 years is only about 1/18,000 as long as that of 238U. Thus the ratio of 234
U to 238
U in a natural sample is equivalent to the ratio of their half-lives. The primary path of production of 234U via nuclear decay is as follows: uranium-238 nuclei emit an alpha particle to become thorium-234. Next, with a short half-life, 234Th nuclei emit a beta particle to become protactinium-234 (234Pa), or more likely a nuclear isomer denoted 234mPa. Finally, 234Pa or 234mPa nuclei emit another beta particle to become 234U nuclei.
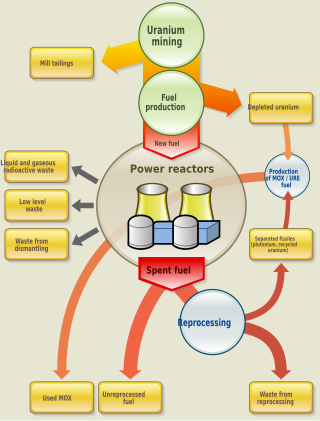
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.

Plutonium-239 is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 is also used for that purpose. Plutonium-239 is also one of the three main isotopes demonstrated usable as fuel in thermal spectrum nuclear reactors, along with uranium-235 and uranium-233. Plutonium-239 has a half-life of 24,110 years.
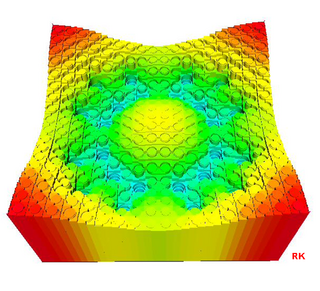
Nuclear reactor physics is the field of physics that studies and deals with the applied study and engineering applications of chain reaction to induce a controlled rate of fission in a nuclear reactor for the production of energy. Most nuclear reactors use a chain reaction to induce a controlled rate of nuclear fission in fissile material, releasing both energy and free neutrons. A reactor consists of an assembly of nuclear fuel, usually surrounded by a neutron moderator such as regular water, heavy water, graphite, or zirconium hydride, and fitted with mechanisms such as control rods which control the rate of the reaction.
The Whiteshell Reactor No. 1, or WR-1, was a Canadian research reactor located at AECL's Whiteshell Laboratories (WNRL) in Manitoba. Originally known as Organic-Cooled Deuterium-Reactor Experiment (OCDRE), it was built to test the concept of a CANDU-type reactor that replaced the heavy water coolant with an oil substance. This had a number of potential advantages in terms of cost and efficiency.

This page discusses each of the main elements in the mixture of fission products produced by nuclear fission of the common nuclear fuels uranium and plutonium. The isotopes are listed by element, in order by atomic number.
In nuclear power technology, burnup is a measure of how much energy is extracted from a primary nuclear fuel source. It is measured as the fraction of fuel atoms that underwent fission in %FIMA or %FIFA as well as, preferably, the actual energy released per mass of initial fuel in gigawatt-days/metric ton of heavy metal (GWd/tHM), or similar units.

The liquid fluoride thorium reactor is a type of molten salt reactor. LFTRs use the thorium fuel cycle with a fluoride-based molten (liquid) salt for fuel. In a typical design, the liquid is pumped between a critical core and an external heat exchanger where the heat is transferred to a nonradioactive secondary salt. The secondary salt then transfers its heat to a steam turbine or closed-cycle gas turbine.
Hybrid nuclear fusion–fission is a proposed means of generating power by use of a combination of nuclear fusion and fission processes.
Reprocessed uranium (RepU) is the uranium recovered from nuclear reprocessing, as done commercially in France, the UK and Japan and by nuclear weapons states' military plutonium production programs. This uranium makes up the bulk of the material separated during reprocessing.
A pressurized heavy-water reactor (PHWR) is a nuclear reactor that uses heavy water (deuterium oxide D2O) as its coolant and neutron moderator. PHWRs frequently use natural uranium as fuel, but sometimes also use very low enriched uranium. The heavy water coolant is kept under pressure to avoid boiling, allowing it to reach higher temperature (mostly) without forming steam bubbles, exactly as for a pressurized water reactor. While heavy water is very expensive to isolate from ordinary water (often referred to as light water in contrast to heavy water), its low absorption of neutrons greatly increases the neutron economy of the reactor, avoiding the need for enriched fuel. The high cost of the heavy water is offset by the lowered cost of using natural uranium and/or alternative fuel cycles. As of the beginning of 2001, 31 PHWRs were in operation, having a total capacity of 16.5 GW(e), representing roughly 7.76% by number and 4.7% by generating capacity of all current operating reactors.
Depleted uranium hexafluoride (DUHF; also referred to as depleted uranium tails, depleted uranium tailings or DUF6) is a byproduct of the processing of uranium hexafluoride into enriched uranium. It is one of the chemical forms of depleted uranium (up to 73-75%), along with depleted triuranium octoxide (up to 25%) and depleted uranium metal (up to 2%). DUHF is 1.7 times less radioactive than uranium hexafluoride and natural uranium.












