
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive and support many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer. When consumed in metabolic processes, it converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP. The human body recycles its own body weight equivalent in ATP each day. It is also a precursor to DNA and RNA, and is used as a coenzyme.

In biochemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology. Protein phosphorylation often activates many enzymes.

Adenosine diphosphate (ADP), also known as adenosine pyrophosphate (APP), is an important organic compound in metabolism and is essential to the flow of energy in living cells. ADP consists of three important structural components: a sugar backbone attached to adenine and two phosphate groups bonded to the 5 carbon atom of ribose. The diphosphate group of ADP is attached to the 5’ carbon of the sugar backbone, while the adenine attaches to the 1’ carbon.

In cell biology, protein kinase A (PKA) is a family of serine-threonine kinase whose activity is dependent on cellular levels of cyclic AMP (cAMP). PKA is also known as cAMP-dependent protein kinase. PKA has several functions in the cell, including regulation of glycogen, sugar, and lipid metabolism. It should not be confused with 5'-AMP-activated protein kinase.

Phosphocreatine, also known as creatine phosphate (CP) or PCr (Pcr), is a phosphorylated form of creatine that serves as a rapidly mobilizable reserve of high-energy phosphates in skeletal muscle, myocardium and the brain to recycle adenosine triphosphate, the energy currency of the cell.
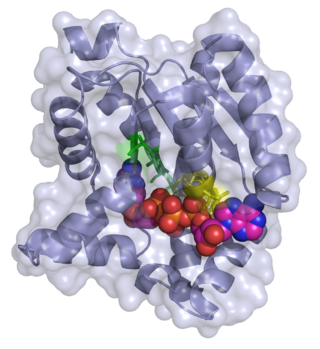
Adenylate kinase is a phosphotransferase enzyme that catalyzes the interconversion of the various adenosine phosphates. By constantly monitoring phosphate nucleotide levels inside the cell, ADK plays an important role in cellular energy homeostasis.
Substrate-level phosphorylation is a metabolism reaction that results in the production of ATP or GTP supported by the energy released from another high-energy bond that leads to phosphorylation of ADP or GDP to ATP or GTP (note that the reaction catalyzed by creatine kinase is not considered as "substrate-level phosphorylation"). This process uses some of the released chemical energy, the Gibbs free energy, to transfer a phosphoryl (PO3) group to ADP or GDP. Occurs in glycolysis and in the citric acid cycle.

A serine/threonine protein kinase is a kinase enzyme, in particular a protein kinase, that phosphorylates the OH group of the amino-acid residues serine or threonine, which have similar side chains. At least 350 of the 500+ human protein kinases are serine/threonine kinases (STK).
In enzymology, an acetylglutamate kinase is an enzyme that catalyzes the chemical reaction:
In enzymology, a beta-adrenergic-receptor kinase is an enzyme that catalyzes the chemical reaction:
In enzymology, a beta-glucoside kinase is an enzyme that catalyzes the chemical reaction
In enzymology, an elongation factor 2 kinase is an enzyme that catalyzes the chemical reaction:
In enzymology, an ethanolamine kinase is an enzyme that catalyzes the chemical reaction
In enzymology, a guanidinoacetate kinase is an enzyme that catalyzes the chemical reaction

Histidine kinases (HK) are multifunctional, and in non-animal kingdoms, typically transmembrane, proteins of the transferase class of enzymes that play a role in signal transduction across the cellular membrane. The vast majority of HKs are homodimers that exhibit autokinase, phosphotransfer, and phosphatase activity. HKs can act as cellular receptors for signaling molecules in a way analogous to tyrosine kinase receptors (RTK). Multifunctional receptor molecules such as HKs and RTKs typically have portions on the outside of the cell that bind to hormone- or growth factor-like molecules, portions that span the cell membrane, and portions within the cell that contain the enzymatic activity. In addition to kinase activity, the intracellular domains typically have regions that bind to a secondary effector molecule or complex of molecules that further propagate signal transduction within the cell. Distinct from other classes of protein kinases, HKs are usually parts of a two-component signal transduction mechanisms in which HK transfers a phosphate group from ATP to a histidine residue within the kinase, and then to an aspartate residue on the receiver domain of a response regulator protein. More recently, the widespread existence of protein histidine phosphorylation distinct from that of two-component histidine kinases has been recognised in human cells. In marked contrast to Ser, Thr and Tyr phosphorylation, the analysis of phosphorylated Histidine using standard biochemical and mass spectrometric approaches is much more challenging, and special procedures and separation techniques are required for their preservation alongside classical Ser, Thr and Tyr phosphorylation on proteins isolated from human cells.
In enzymology, a lombricine kinase is an enzyme that catalyzes the chemical reaction
In enzymology, a protein-histidine pros-kinase is an enzyme that catalyzes the chemical reaction
In enzymology, a protein-histidine tele-kinase is an enzyme that catalyzes the chemical reaction

Protein phosphorylation is a reversible post-translational modification of proteins in which an amino acid residue is phosphorylated by a protein kinase by the addition of a covalently bound phosphate group. Phosphorylation alters the structural conformation of a protein, causing it to become either activated or deactivated, or otherwise modifying its function. Approximately 13000 human proteins have sites that are phosphorylated.

Protein Arginine Phosphatase (PAPs), also known as Phosphoarginine Phosphatase, is an enzyme that catalyzes the dephosphorylation of phosphoarginine residues in proteins. Protein phosphatases (PPs) are "obligatory heteromers" made up of two maximum catalytic subunits attached to a non-catalytic subunit. Arginine modification is a post-translational protein modification in gram-positive bacteria. McsB and YwIE were recently identified as phosphorylating enzymes in Bacillus Subtilis (B.Subtilis). YwIE was thought to be a protein-tyrosine-phosphatase, and McsB a tyrosine-kinase, however in 2012 Elsholz et al. showed that McsB is a protein-arginine-kinase (PAK) and YwlE is a phosphatase-arginine-phosphatase (PAP).










