
Boron nitride is a heat and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite and may even be harder than the cubic form.

A ceramic is a solid material comprising an inorganic compound of metal, non-metal or metalloid atoms primarily held in ionic and covalent bonds. Common examples are earthenware, porcelain, and brick.

Piezoelectricity is the electric charge that accumulates in certain solid materials in response to applied mechanical stress. The word piezoelectricity means electricity resulting from pressure and latent heat. It is derived from the Greek word πιέζειν; piezein, which means to squeeze or press, and ἤλεκτρον ēlektron, which means amber, an ancient source of electric charge. French physicists Jacques and Pierre Curie discovered piezoelectricity in 1880.

High-temperature superconductors are materials that behave as superconductors at unusually high temperatures. The first high-Tc superconductor was discovered in 1986 by IBM researchers Georg Bednorz and K. Alex Müller, who were awarded the 1987 Nobel Prize in Physics "for their important break-through in the discovery of superconductivity in ceramic materials".

A perovskite is any material with the same type of crystal structure as calcium titanium oxide (CaTiO3), known as the perovskite structure, or XIIA2+VIB4+X2−3 with the oxygen in the edge centers. Perovskites take their name from the mineral, which was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and is named after Russian mineralogist L. A. Perovski (1792–1856). The general chemical formula for perovskite compounds is ABX3, where 'A' and 'B' are two cations of very different sizes, and X is an anion that bonds to both. The 'A' atoms are larger than the 'B' atoms. The ideal cubic structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedral coordination. The relative ion size requirements for stability of the cubic structure are quite stringent, so slight buckling and distortion can produce several lower-symmetry distorted versions, in which the coordination numbers of A cations, B cations or both are reduced.

Yttrium barium copper oxide (YBCO) is a family of crystalline chemical compounds, famous for displaying high-temperature superconductivity. It includes the first material ever discovered to become superconducting above the boiling point of liquid nitrogen (77 K) at about 90 K. Many YBCO compounds have the general formula YBa2Cu3O7−x (also known as Y123), although materials with other Y:Ba:Cu ratios exist, such as YBa2Cu4Oy (Y124) or Y2Ba4Cu7Oy (Y247).

An opacifier is a substance added to a material in order to make the ensuing system opaque. An example of a chemical opacifier is titanium dioxide (TiO2), which is used as an opacifier in paints, in paper, and in plastics. It has very high refraction index (rutile modification 2.7 and anatase modification 2.55) and optimum refraction is obtained with crystals about 225 nanometers. Impurities in the crystal alter the optical properties. It is also used to opacify ceramic glazes and milk glass; bone ash is also used.
Electroceramics is a class of ceramic materials used primarily for their electrical properties.

A ferrite is a ceramic material made by mixing and firing large proportions iron(III) oxide (Fe2O3, rust) blended with small proportions of one or more additional metallic elements, such as barium, manganese, nickel, and zinc. They are both electrically non-conductive, meaning that they are insulators, and ferrimagnetic, meaning they can easily be magnetized or attracted to a magnet. Ferrites can be divided into two families based on their resistance to being demagnetized (magnetic coercivity).

Lanthanum oxide, chemical formula La2O3, is an inorganic compound containing the rare earth element lanthanum and oxygen. It is used in some ferroelectric materials, as a component of optical materials, and is a feedstock for certain catalysts, among other uses.
Barium ferrite, abbreviated BaFe, BaM, is the chemical compound with the formula BaFe12O19. This and related ferrite materials are components in magnetic stripe cards and loudspeaker magnets. BaFe is described as Ba2+(Fe3+)12(O2−)19. The Fe3+ centers are ferromagnetically coupled. This area of technology is usually considered to be an application of the related fields of materials science and solid state chemistry.

Barium titanate is an inorganic compound with chemical formula BaTiO3. Barium titanate appears white as a powder and is transparent when prepared as large crystals. It is a ferroelectric ceramic material that exhibits the photorefractive effect and piezoelectric properties. It is used in capacitors, electromechanical transducers and nonlinear optics.
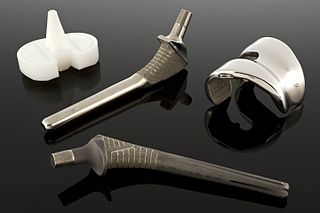
A biomaterial is any substance that has been engineered to interact with biological systems for a medical purpose - either a therapeutic or a diagnostic one. As a science, biomaterials is about fifty years old. The study of biomaterials is called biomaterials science or biomaterials engineering. It has experienced steady and strong growth over its history, with many companies investing large amounts of money into the development of new products. Biomaterials science encompasses elements of medicine, biology, chemistry, tissue engineering and materials science.
Barium borate is an inorganic compound, a borate of barium with a chemical formula BaB2O4 or Ba(BO2)2. It is available as a hydrate or dehydrated form, as white powder or colorless crystals. The crystals exist in the high-temperature α phase and low-temperature β phase, abbreviated as BBO; both phases are birefringent, and BBO is a common nonlinear optical material.
Mechanical metamaterials are artificial structures with mechanical properties defined by their structure rather than their composition. They can be seen as a counterpart to the rather well-known family of optical metamaterials and include acoustic metamaterials as a special case of vanishing shear. Their mechanical properties can be designed to have values which cannot be found in nature.
Barium orthotitanate is the inorganic compound with the chemical formula Ba2TiO4. It is a colourless solid that is of interest because of its relationship to barium titanate, a useful electroceramic.
A. R. Forouhi and I. Bloomer deduced dispersion equations for the refractive index, n, and extinction coefficient, k, which were published in 1986 and 1988. The 1986 publication relates to amorphous materials, while the 1988 publication relates to crystalline. Subsequently, in 1991, their work was included as a chapter in “The Handbook of Optical Constants”. The Forouhi–Bloomer dispersion equations describe how photons of varying energies interact with thin films. When used with a spectroscopic reflectometry tool, the Forouhi–Bloomer dispersion equations specify n and k for amorphous and crystalline materials as a function of photon energy E. Values of n and k as a function of photon energy, E, are referred to as the spectra of n and k, which can also be expressed as functions of wavelength of light, λ, since E = hc/λ. The symbol h represents Planck’s constant and c, the speed of light in vacuum. Together, n and k are often referred to as the “optical constants” of a material.

Bismuth molecules have the ability to form trivalent or pentavalent compounds. Lead bismuthate itself has only been discovered in recent years in the laboratory as it is not naturally occurring. Lead bismuthate forms a pentavalent structure, significantly different from the regular ionic interactions of sodium bismuthate, but similar to that of strontium bismuthate. In the structure, six oxygen atoms are coordinated octahedrally to both the bismuth and lead atoms. The bismuth and oxygen atoms form negatively charged layers by creating repeating octahedral geometries. The positively charged lead atoms are then disbursed within the layers, forming a hexagonal unit cell, with a lead atom in each of the corners. The density of the crystal is 9.18 g/cm3. The formula weight is 233.99 g/mol. The volume of the crystal structure unit is 169.26 A3. Lattice parameters (a) is 5.321 angstroms.
Nickel forms a series of mixed oxide compounds which are commonly called nickelates. A nickelate is an anion containing nickel or a salt containing a nickelate anion, or a double compound containing nickel bound to oxygen and other elements. Nickel can be in different or even mixed oxidation states, ranging from +1, +2, +3 to +4. The anions can contain a single nickel ion, or multiple to form a cluster ion. The solid mixed oxide compounds are often ceramics, but can also be metallic. They have a variety of electrical and magnetic properties. Rare-earth elements form a range of perovskite nickelates, in which the properties vary systematically as the rare-earth element changes. Fine tuning of properties is achievable with mixtures of elements, applying stress or pressure, or varying the physical form.
The nitronickelates are a class of chemical compounds containing a nickel atom complexed by nitro groups, -NO2. Nickel can be in a +2 or +3 oxidation state. There can be five (pentanitronickelates), or six, (hexanitronickelates) nitro groups per nickel atom. The can be considered the double nitrites of nickel nitrite.














