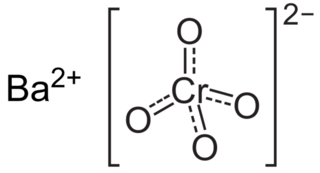Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.

Oxalic acid is an organic acid with the systematic name ethanedioic acid and formula HO2C−CO2H. It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early investigators isolated oxalic acid from flowering plants of the genus Oxalis, commonly known as wood-sorrels. It occurs naturally in many foods. Excessive ingestion of oxalic acid or prolonged skin contact can be dangerous.
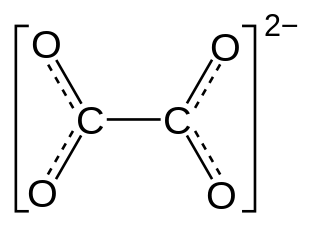
Oxalate (IUPAC: ethanedioate) is an anion with the formula C2O42−. This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (Na2C2O4), and several esters such as dimethyl oxalate (C2O4(CH3)2). It is a conjugate base of oxalic acid. At neutral pH in aqueous solution, oxalic acid converts completely to oxalate.

Barium sulfate (or sulphate) is the inorganic compound with the chemical formula BaSO4. It is a white crystalline solid that is odorless and insoluble in water. It occurs as the mineral barite, which is the main commercial source of barium and materials prepared from it. The white opaque appearance and its high density are exploited in its main applications.

Flash powder is a pyrotechnic composition, a mixture of oxidizer and metallic fuel, which burns quickly and produces a loud noise regardless of confinement. It is widely used in theatrical pyrotechnics and fireworks and was once used for flashes in photography.

Barium chloride is an inorganic compound with the formula BaCl2. It is one of the most common water-soluble salts of barium. Like most other water-soluble barium salts, it is a white powder, highly toxic, and imparts a yellow-green coloration to a flame. It is also hygroscopic, converting to the dihydrate BaCl2·2H2O, which are colourless crystals with a bitter salty taste. It has limited use in the laboratory and industry.

Chloric acid, HClO3, is an oxoacid of chlorine, and the formal precursor of chlorate salts. It is a strong acid (pKa ≈ −2.7 (***note: pKa not in agreement with properties in chem box at right)) and an oxidizing agent.

Barium nitrate is the inorganic compound with the chemical formula Ba(NO3)2. It, like most barium salts, is colorless, toxic, and water-soluble. It burns with a green flame and is an oxidizer; the compound is commonly used in pyrotechnics.

A permanganate is a chemical compound containing the manganate(VII) ion, MnO−
4, the conjugate base of permanganic acid. Because the manganese atom is in the +7 oxidation state, the permanganate(VII) ion is a strong oxidizing agent. The ion is a transition metal oxo complex with tetrahedral geometry. Permanganate solutions are purple in color and are stable in neutral or slightly alkaline media. The exact chemical reaction is dependent upon the organic contaminants present and the oxidant utilized. For example, trichloroethane (C2H3Cl3) is oxidized by permanganate ions to form carbon dioxide (CO2), manganese dioxide (MnO2), hydrogen ions (H+), and chloride ions (Cl−).
A pyrotechnic colorant is a chemical compound which causes a flame to burn with a particular color. These are used to create the colors in pyrotechnic compositions like fireworks and colored fires. The color-producing species are usually created from other chemicals during the reaction. Metal salts are commonly used; elemental metals are used rarely.

Sodium oxalate, or disodium oxalate, is the sodium salt of oxalic acid with the formula Na2C2O4. It is a white, crystalline, odorless solid, that decomposes above 290 °C.
Americium dioxide (AmO2) is a black compound of americium. In the solid state AmO2 adopts the fluorite, CaF2 structure. It is used as a source of alpha particles.
A pyrotechnic composition is a substance or mixture of substances designed to produce an effect by heat, light, sound, gas/smoke or a combination of these, as a result of non-detonative self-sustaining exothermic chemical reactions. Pyrotechnic substances do not rely on oxygen from external sources to sustain the reaction.
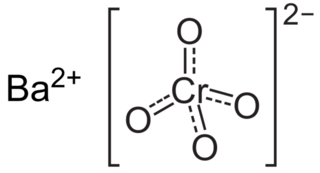
Barium chromate, named barium tetraoxochromate(VI) by the IUPAC, is a yellow sand like powder with the formula BaCrO4. It is a known oxidizing agent and produces a green flame when heated, a result of the barium ions.

Barium chlorate, Ba(ClO3)2, is the barium salt of chloric acid. It is a white crystalline solid, and like all soluble barium compounds, irritant and toxic. It is sometimes used in pyrotechnics to produce a green color. It also finds use in the production of chloric acid.

Strontium peroxide is an inorganic compound with the formula SrO2 that exists in both anhydrous and octahydrate form, both of which are white solids. The anhydrous form adopts a structure similar to that of calcium carbide.

Barium bromide is the chemical compound with the formula BaBr2. It is ionic in nature.

Barium ferrate is the chemical compound of formula BaFeO4. This is a rare compound containing iron in the +6 oxidation state. The ferrate(VI) ion has two unpaired electrons, making it paramagnetic. It is isostructural with BaSO4, and contains the tetrahedral [FeO4]2− anion.
Barium perchlorate is a powerful oxidizing agent, with the formula Ba(ClO4)2. It is used in the pyrotechnic industry.
Chromium(II) oxalate is an inorganic compound with the chemical formula CrC2O4.