In chemistry, titanate usually refers to inorganic compounds composed of titanium oxides, or oxides containing the titanium element. Together with niobate, titanate salts form the Perovskite group.

Yttrium barium copper oxide (YBCO) is a family of crystalline chemical compounds that display high-temperature superconductivity; it includes the first material ever discovered to become superconducting above the boiling point of liquid nitrogen [77 K ] at about 93 K.

Calcium sulfate (or calcium sulphate) is the inorganic compound with the formula CaSO4 and related hydrates. In the form of γ-anhydrite (the anhydrous form), it is used as a desiccant. One particular hydrate is better known as plaster of Paris, and another occurs naturally as the mineral gypsum. It has many uses in industry. All forms are white solids that are poorly soluble in water. Calcium sulfate causes permanent hardness in water.
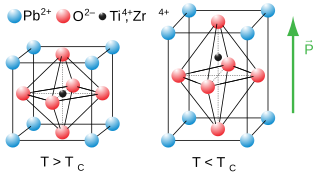
Lead zirconate titanate, also called lead zirconium titanate and commonly abbreviated as PZT, is an inorganic compound with the chemical formula Pb[ZrxTi1−x]O3(0 ≤ x ≤ 1).. It is a ceramic perovskite material that shows a marked piezoelectric effect, meaning that the compound changes shape when an electric field is applied. It is used in a number of practical applications such as ultrasonic transducers and piezoelectric resonators. It is a white to off-white solid.

Celsian is an uncommon feldspar mineral, barium aluminosilicate, BaAl2Si2O8. The mineral occurs in contact metamorphic rocks with significant barium content. Its crystal system is monoclinic, and it is white, yellow, or transparent in appearance. In pure form, it is transparent. Synthetic barium aluminosilicate is used as a ceramic in dental fillings and other applications.

Barium titanate (BTO) is an inorganic compound with chemical formula BaTiO3. Barium titanate appears white as a powder and is transparent when prepared as large crystals. It is a ferroelectric, pyroelectric, and piezoelectric ceramic material that exhibits the photorefractive effect. It is used in capacitors, electromechanical transducers and nonlinear optics.

Titanium isopropoxide, also commonly referred to as titanium tetraisopropoxide or TTIP, is a chemical compound with the formula Ti{OCH(CH3)2}4. This alkoxide of titanium(IV) is used in organic synthesis and materials science. It is a diamagnetic tetrahedral molecule. Titanium isopropoxide is a component of the Sharpless epoxidation, a method for the synthesis of chiral epoxides.

Lithium titanates are chemical compounds of lithium, titanium and oxygen. They are mixed oxides and belong to the titanates. The most important lithium titanates are:

Barium borate is an inorganic compound, a borate of barium with a chemical formula BaB2O4 or Ba(BO2)2. It is available as a hydrate or dehydrated form, as white powder or colorless crystals. The crystals exist in the high-temperature α phase and low-temperature β phase, abbreviated as BBO; both phases are birefringent, and BBO is a common nonlinear optical material.

Calcium titanate is an inorganic compound with the chemical formula CaTiO3. As a mineral, it is called perovskite, named after Russian mineralogist, L. A. Perovski (1792-1856). It is a colourless, diamagnetic solid, although the mineral is often coloured owing to impurities.
Barium permanganate is a chemical compound, with the formula Ba(MnO4)2. It forms violet to brown crystals that are sparingly soluble in water.

A metaborate is a borate anion consisting of boron and oxygen, with empirical formula BO−2. Metaborate also refers to any salt or ester of such anion. Metaborate is one of the boron's oxyanions. Metaborates can be monomeric, oligomeric or polymeric.
Langbeinites are a family of crystalline substances based on the structure of langbeinite with general formula M2M'2(SO4)3, where M is a large univalent cation, and M' is a small divalent cation. The sulfate group, SO2−4, can be substituted by other tetrahedral anions with a double negative charge such as tetrafluoroberyllate, selenate, chromate, molybdate, or tungstates. Although monofluorophosphates are predicted, they have not been described. By redistributing charges other anions with the same shape such as phosphate also form langbeinite structures. In these the M' atom must have a greater charge to balance the extra three negative charges.

Abnormal or discontinuous grain growth, also referred to as exaggerated or secondary recrystallisation grain growth, is a grain growth phenomenon in which certain energetically favorable grains (crystallites) grow rapidly in a matrix of finer grains, resulting in a bimodal grain-size distribution.
Nickel forms a series of mixed oxide compounds which are commonly called nickelates. A nickelate is an anion containing nickel or a salt containing a nickelate anion, or a double compound containing nickel bound to oxygen and other elements. Nickel can be in different or even mixed oxidation states, ranging from +1, +2, +3 to +4. The anions can contain a single nickel ion, or multiple to form a cluster ion. The solid mixed oxide compounds are often ceramics, but can also be metallic. They have a variety of electrical and magnetic properties. Rare-earth elements form a range of perovskite nickelates, in which the properties vary systematically as the rare-earth element changes. Fine tuning of properties is achievable with mixtures of elements, applying stress or pressure, or varying the physical form.

The Nickel oxyacid salts are a class of chemical compounds of nickel with an oxyacid. The compounds include a number of minerals and industrially important nickel compounds.
Anthony Roy West FRSE, FRSC, FInstP, FIMMM is a British chemist and materials scientist, and Professor of Electroceramics and Solid State Chemistry at the Department of Materials Science and Engineering at the University of Sheffield.
Robert Day Shannon is a retired research chemist formerly at DuPont de Nemours, Inc.
Protactinium compounds are compounds containing the element protactinium. These compounds usually have protactinium in the +5 oxidation state, although these compounds can also exist in the +2, +3 and +4 oxidation states.












