In chemistry, hydronium (hydroxonium in traditional British English) is the common name for the cation [H3O]+, also written as H3O+, the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid is dissolved in water, as Arrhenius acid molecules in solution give up a proton (a positive hydrogen ion, H+) to the surrounding water molecules (H2O). In fact, acids must be surrounded by more than a single water molecule in order to ionize, yielding aqueous H+ and conjugate base. Three main structures for the aqueous proton have garnered experimental support: the Eigen cation, which is a tetrahydrate, H3O+(H2O)3, the Zundel cation, which is a symmetric dihydrate, H+(H2O)2, and the Stoyanov cation, an expanded Zundel cation, which is a hexahydrate: H+(H2O)2(H2O)4. Spectroscopic evidence from well-defined IR spectra overwhelmingly supports the Stoyanov cation as the predominant form. For this reason, it has been suggested that wherever possible, the symbol H+(aq) should be used instead of the hydronium ion.

Titanic acid is a general name for a family of chemical compounds of the elements titanium, hydrogen, and oxygen, with the general formula [TiOx(OH)4−2x]n. Various simple titanic acids have been claimed, mainly in the older literature. No crystallographic and little spectroscopic support exists for these materials. Some older literature refers to TiO2 as titanic acid, and the dioxide forms an unstable hydrate when TiCl4 hydrolyzes.

Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is a volatile liquid. Upon contact with humid air, it forms thick clouds of titanium dioxide and hydrochloric acid, a reaction that was formerly exploited for use in smoke machines. It is sometimes referred to as “tickle” or “tickle 4”, as a phonetic representation of the symbols of its molecular formula.

Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions.
Manganese(III) fluoride (also known as Manganese trifluoride) is the inorganic compound with the formula MnF3. This red/purplish solid is useful for converting hydrocarbons into fluorocarbons, i.e., it is a fluorination agent. It forms a hydrate and many derivatives.
An inorganic nonaqueous solvent is a solvent other than water, that is not an organic compound. These solvents are used in chemical research and industry for reactions that cannot occur in aqueous solutions or require a special environment. Inorganic nonaqueous solvents can be classified into two groups, protic solvents and aprotic solvents. Early studies on inorganic nonaqueous solvents evaluated ammonia, hydrogen fluoride, sulfuric acid, as well as more specialized solvents, hydrazine, and selenium oxychloride.

18-Crown-6 is an organic compound with the formula [C2H4O]6 and the IUPAC name of 1,4,7,10,13,16-hexaoxacyclooctadecane. It is a white, hygroscopic crystalline solid with a low melting point. Like other crown ethers, 18-crown-6 functions as a ligand for some metal cations with a particular affinity for potassium cations (binding constant in methanol: 106 M−1). The point group of 18-crown-6 is S6. The dipole moment of 18-crown-6 varies in different solvent and under different temperature. Under 25 °C, the dipole moment of 18-crown-6 is 2.76 ± 0.06 D in cyclohexane and 2.73 ± 0.02 in benzene. The synthesis of the crown ethers led to the awarding of the Nobel Prize in Chemistry to Charles J. Pedersen.

Uranyl chloride refers to inorganic compounds with the formula UO2Cl2(H2O)n where n = 0, 1, or 3. These are yellow-colored salts.

Hexafluorosilicic acid is an inorganic compound with the chemical formula H
2SiF
6. Aqueous solutions of hexafluorosilicic acid consist of salts of the cation and hexafluorosilicate anion. These salts and their aqueous solutions are colorless.
Titanium(III) chloride is the inorganic compound with the formula TiCl3. At least four distinct species have this formula; additionally hydrated derivatives are known. TiCl3 is one of the most common halides of titanium and is an important catalyst for the manufacture of polyolefins.
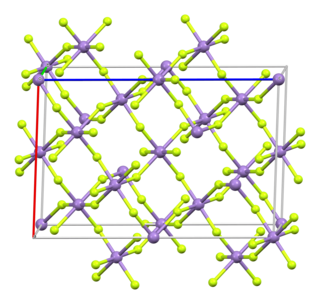
Titanium(IV) fluoride is the inorganic compound with the formula TiF4. It is a white hygroscopic solid. In contrast to the other tetrahalides of titanium, it adopts a polymeric structure. In common with the other tetrahalides, TiF4 is a strong Lewis acid.
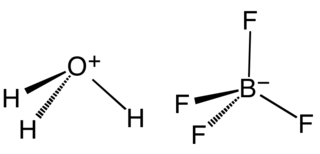
Fluoroboric acid or tetrafluoroboric acid is an inorganic compound with the simplified chemical formula H+[BF4]−. Unlike other strong acids like H2SO4 or HClO4, the pure tetrafluoroboric acid does not exist. The term "fluoroboric acid" refers to a range of chemical compounds, depending on the solvent. The H+ in the simplified formula of fluoroboric acid represents the solvated proton. The solvent can be any suitable Lewis base. For instance, if the solvent is water, fluoroboric acid can be represented by the formula [H3O]+[BF4]−, although more realistically, several water molecules solvate the proton: [H(H2O)n]+[BF4]−. The ethyl ether solvate is also commercially available, where the fluoroboric acid can be represented by the formula [H( 2O)n]+[BF4]−, where n is most likely 2.

Chloroauric acid is an inorganic compound with the chemical formula H[AuCl4]. It forms hydrates H[AuCl4]·nH2O. Both the trihydrate and tetrahydrate are known. Both are orange-yellow solids consisting of the planar [AuCl4]− anion. Often chloroauric acid is handled as a solution, such as those obtained by dissolution of gold in aqua regia. These solutions can be converted to other gold complexes or reduced to metallic gold or gold nanoparticles.

Sodium monothiophosphate, or sodium phosphorothioate, is an inorganic compound with the chemical formula Na3PO3S. It is a sodium salt of monothiophosphoric acid (H3PO3S). Sodium monothiophosphate forms hydrates Na3PO3S·xH2O. The anhydrous form and all hydrates are white solids. The anhydrous salt (x = 0) (Na3PO3S) decomposes without melting at 120-125 °C. More common is the dodecahydrate (Na3PO3S·12H2O). A nonahydrate is also known (Na3PO3S·9H2O).
Cobalt(II) cyanide is the inorganic compound with the formula Co(CN)2. It is coordination polymer that has attracted intermittent attention over many years in the area of inorganic synthesis and homogeneous catalysis.

Ammonium hexafluorotitanate is the inorganic compound with the chemical formula (NH4)2[TiF6]. A colorless salt, the compound consists of ammonium ions and the hexafluorotitanate dianion. It is encountered in the extraction of titanium from its principal ore ilmenite: the ore is treated with excess ammonium fluoride:

Titanium nitrate is the inorganic compound with formula Ti(NO3)4. It is a colorless, diamagnetic solid that sublimes readily. It is an unusual example of a volatile binary transition metal nitrate. Ill defined species called titanium nitrate are produced upon dissolution of titanium or its oxides in nitric acid.
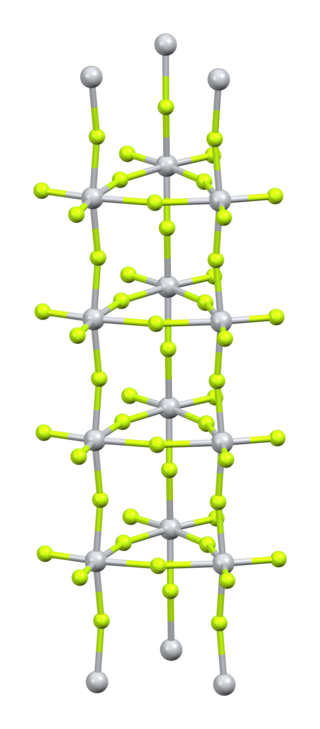
Platinum tetrafluoride is the inorganic compound with the chemical formula PtF
4. In the solid state, the compound features platinum(IV) in octahedral coordination geometry.
Nickel compounds are chemical compounds containing the element nickel which is a member of the group 10 of the periodic table. Most compounds in the group have an oxidation state of +2. Nickel is classified as a transition metal with nickel(II) having much chemical behaviour in common with iron(II) and cobalt(II). Many salts of nickel(II) are isomorphous with salts of magnesium due to the ionic radii of the cations being almost the same. Nickel forms many coordination complexes. Nickel tetracarbonyl was the first pure metal carbonyl produced, and is unusual in its volatility. Metalloproteins containing nickel are found in biological systems.

The hexafluoroarsenate anion is a chemical species with formula AsF−6. Hexafluoroarsenate is relatively inert, being the conjugate base of the notional superacid hexafluoroarsenic acid.















