
Europium is a chemical element; it has symbol Eu and atomic number 63. Europium is a silvery-white metal of the lanthanide series that reacts readily with air to form a dark oxide coating. It is the most chemically reactive, least dense, and softest of the lanthanide elements. It is soft enough to be cut with a knife. Europium was isolated in 1901 and named after the continent of Europe. Europium usually assumes the oxidation state +3, like other members of the lanthanide series, but compounds having oxidation state +2 are also common. All europium compounds with oxidation state +2 are slightly reducing. Europium has no significant biological role and is relatively non-toxic compared to other heavy metals. Most applications of europium exploit the phosphorescence of europium compounds. Europium is one of the rarest of the rare-earth elements on Earth.
Ferroelectricity is a characteristic of certain materials that have a spontaneous electric polarization that can be reversed by the application of an external electric field. All ferroelectrics are also piezoelectric and pyroelectric, with the additional property that their natural electrical polarization is reversible. The term is used in analogy to ferromagnetism, in which a material exhibits a permanent magnetic moment. Ferromagnetism was already known when ferroelectricity was discovered in 1920 in Rochelle salt by Joseph Valasek. Thus, the prefix ferro, meaning iron, was used to describe the property despite the fact that most ferroelectric materials do not contain iron. Materials that are both ferroelectric and ferromagnetic are known as multiferroics.

Strontium titanate is an oxide of strontium and titanium with the chemical formula SrTiO3. At room temperature, it is a centrosymmetric paraelectric material with a perovskite structure. At low temperatures it approaches a ferroelectric phase transition with a very large dielectric constant ~104 but remains paraelectric down to the lowest temperatures measured as a result of quantum fluctuations, making it a quantum paraelectric. It was long thought to be a wholly artificial material, until 1982 when its natural counterpart—discovered in Siberia and named tausonite—was recognised by the IMA. Tausonite remains an extremely rare mineral in nature, occurring as very tiny crystals. Its most important application has been in its synthesized form wherein it is occasionally encountered as a diamond simulant, in precision optics, in varistors, and in advanced ceramics.

Barium titanate (BTO) is an inorganic compound with chemical formula BaTiO3. Barium titanate appears white as a powder and is transparent when prepared as large crystals. It is a ferroelectric, pyroelectric, and piezoelectric ceramic material that exhibits the photorefractive effect. It is used in capacitors, electromechanical transducers and nonlinear optics.

Lanthanum strontium manganite (LSM or LSMO) is an oxide ceramic material with the general formula La1−xSrxMnO3, where x describes the doping level.
The Burns temperature, Td, is the temperature where a ferroelectric material, previously in paraelectric state, starts to present randomly polarized nanoregions, that are polar precursor clusters. This behaviour is typical of several, but not all, ferroelectric materials, and was observed in lead titanate (PbTiO3), potassium niobate (KNbO3), lead lanthanum zirconate titanate (PLZT), lead magnesium niobate (PMN), lead zinc niobate (PZN), K2Sr4(NbO3)10, and strontium barium niobate (SBN), Na1/2Bi1/2O3 (NBT).
A superinsulator is a material that at low but finite temperatures does not conduct electricity, i.e. has an infinite resistance so that no electric current passes through it. The phenomenon of superinsulation can be regarded as an exact dual to superconductivity.

Lithium titanates are chemical compounds of lithium, titanium and oxygen. They are mixed oxides and belong to the titanates. The most important lithium titanates are:
Solid hydrogen is the solid state of the element hydrogen, achieved by decreasing the temperature below hydrogen's melting point of 14.01 K. It was collected for the first time by James Dewar in 1899 and published with the title "Sur la solidification de l'hydrogène" in the Annales de Chimie et de Physique, 7th series, vol. 18, Oct. 1899. Solid hydrogen has a density of 0.086 g/cm3 making it one of the lowest-density solids.

Perovskite (pronunciation: ) is a calcium titanium oxide mineral composed of calcium titanate (chemical formula CaTiO3). Its name is also applied to the class of compounds which have the same type of crystal structure as CaTiO3, known as the perovskite structure, which has a general chemical formula A2+B4+(X2−)3. Many different cations can be embedded in this structure, allowing the development of diverse engineered materials.
Spinons are one of three quasiparticles, along with holons and orbitons, that electrons in solids are able to split into during the process of spin–charge separation, when extremely tightly confined at temperatures close to absolute zero. The electron can always be theoretically considered as a bound state of the three, with the spinon carrying the spin of the electron, the orbiton carrying the orbital location and the holon carrying the charge, but in certain conditions they can behave as independent quasiparticles.

Superconducting wires are electrical wires made of superconductive material. When cooled below their transition temperatures, they have zero electrical resistance. Most commonly, conventional superconductors such as niobium–titanium are used, but high-temperature superconductors such as YBCO are entering the market.
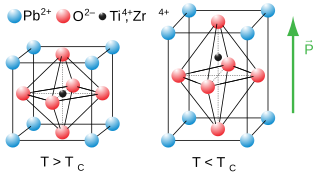
Lead(II) titanate is an inorganic compound with the chemical formula PbTiO3. It is the lead salt of titanic acid. Lead(II) titanate is a yellow powder that is insoluble in water.
Iron(II) selenide refers to a number of inorganic compounds of ferrous iron and selenide (Se2−). The phase diagram of the system Fe–Se reveals the existence of several non-stoichiometric phases between ~49 at. % Se and ~53 at. % Fe, and temperatures up to ~450 °C. The low temperature stable phases are the tetragonal PbO-structure (P4/nmm) β-Fe1−xSe and α-Fe7Se8. The high temperature phase is the hexagonal, NiAs structure (P63/mmc) δ-Fe1−xSe. Iron(II) selenide occurs naturally as the NiAs-structure mineral achavalite.
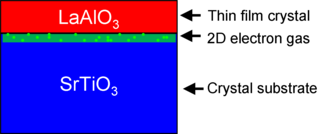
The interface between lanthanum aluminate (LaAlO3) and strontium titanate (SrTiO3) is a notable materials interface because it exhibits properties not found in its constituent materials. Individually, LaAlO3 and SrTiO3 are non-magnetic insulators, yet LaAlO3/SrTiO3 interfaces can exhibit electrical metallic conductivity, superconductivity, ferromagnetism, large negative in-plane magnetoresistance, and giant persistent photoconductivity. The study of how these properties emerge at the LaAlO3/SrTiO3 interface is a growing area of research in condensed matter physics.
Lanthanum aluminate is an inorganic compound with the formula LaAlO3, often abbreviated as LAO. It is an optically transparent ceramic oxide with a distorted perovskite structure.
Sodium bismuth titanate or bismuth sodium titanium oxide (NBT or BNT) is a solid inorganic compound of sodium, bismuth, titanium and oxygen with the chemical formula of Na0.5Bi0.5TiO3 or Bi0.5Na0.5TiO3. This compound adopts the perovskite structure.
Europium(III) chromate is a chemical compound composed of europium, chromium and oxygen with europium in the +3 oxidation state, chromium in the +5 oxidation state and oxygen in the -2 oxidation state. It has the chemical formula of EuCrO4.
Europium(III) oxalate (Eu2(C2O4)3) is a chemical compound of europium and oxalic acid. There are different hydrates including the decahydrate, hexahydrate and tetrahydrate. Europium(II) oxalate is also known.

Europium(III) phosphate is one of the phosphates of europium, with the chemical formula of EuPO4. Other phosphates include europium(II) phosphate (Eu3(PO4)2) and europium(II,III) phosphate (Eu3Eu(PO4)3).











