Related Research Articles
Aluminium carbonate (Al2(CO3)3), is a carbonate of aluminium. It is not well characterized; one authority says that simple carbonates of aluminium are not known. However related compounds are known, such as the basic sodium aluminium carbonate mineral dawsonite (NaAlCO3(OH)2) and hydrated basic aluminium carbonate minerals scarbroite (Al5(CO3)(OH)13•5(H2O)) and hydroscarbroite (Al14(CO3)3(OH)36•nH2O).
Fluorooxoborate is one of a series of anions or salts that contain boron linked to both oxygen and fluorine. Several structures are possible, rings, or chains. They contain [BOxF4−x](x+1)− units BOF32− BO2F23−, or BO3F14−. In addition there can be borate BO3 triangles and BO4 tetrahedrons. These can then be linked by sharing oxygen atoms, and when they do that, the negative charge is reduced. They are distinct from the fluoroborates in which fluorine is bonded to the metals rather than the boron atoms. For example, KBBF, KBe2BO3F2 is a fluoroborate and has more fluorine and oxygen than can be accommodated by the boron atom.
The borate fluorides or fluoroborates are compounds containing borate or complex borate ions along with fluoride ions that form salts with cations such as metals. They are in the broader category of mixed anion compounds. They are not to be confused with tetrafluoroborates (BF4) or the fluorooxoborates which have fluorine bonded to boron.
Mixed-anion compounds, heteroanionic materials or mixed-anion materials are chemical compounds containing cations and more than one kind of anion. The compounds contain a single phase, rather than just a mixture.
The iodate fluorides are chemical compounds which contain both iodate and fluoride anions (IO3− and F−). In these compounds fluorine is not bound to iodine as it is in fluoroiodates.
The sulfate fluorides are double salts that contain both sulfate and fluoride anions. They are in the class of mixed anion compounds. Some of these minerals are deposited in fumaroles.
The borosulfates are heteropoly anion compounds which have sulfate groups attached to boron atoms. Other possible terms are sulfatoborates or boron-sulfur oxides. The ratio of sulfate to borate reflects the degree of condensation. With [B(SO4)4]5- there is no condensation, each ion stands alone. In [B(SO4)3]3- the anions are linked into a chain, a chain of loops, or as [B2(SO4)6]6− in a cycle. Finally in [B(SO4)2]− the sulfate and borate tetrahedra are all linked into a two or three-dimensional network. These arrangements of oxygen around boron and sulfur can have forms resembling silicates. The first borosulfate to be discovered was K5[B(SO4)4] in 2012. Over 75 unique compounds are known.
The borophosphates are mixed anion compounds containing borate and phosphate anions, which may be joined together by a common oxygen atom. Compounds that contain water or hydroxy groups can also be included in the class of compounds.
The borotellurates are heteropoly anion compounds which have tellurate groups attached to boron atoms. The ratio of tellurate to borate reflects the degree of condensation. In [TeO4(BO3)2]8- the anions are linked into a chain. In [TeO2(BO3)4]10− the structure is zero dimensional with isolated anions. These arrangements of oxygen around boron and tellurium can have forms resembling silicates. The first borotellurates to be discovered were the mixed sodium rare earth compounds in 2015.
Borate sulfates are mixed anion compounds containing separate borate and sulfate anions. They are distinct from the borosulfates where the borate is linked to a sulfate via a common oxygen atom.
Borate nitrates are mixed anion compounds containing separate borate and nitrate anions. They are distinct from the boronitrates where the borate is linked to a nitrate via a common oxygen atom.
Borate sulfides are chemical mixed anion compounds that contain any kind of borate and sulfide ions. They are distinct from thioborates in which sulfur atoms replace oxygen in borates. There are also analogous borate selenides, with selenium ions instead of sulfur.
The borate chlorides are chemical compounds that contain both borate ions and chloride ions. They are mixed anion compounds. Many of them are minerals. Those minerals that crystallise with water (hydrates) may be found in evaporite deposits formed when mineral water has dried out.
Borate phosphates are mixed anion compounds containing separate borate and phosphate anions. They are distinct from the borophosphates where the borate is linked to a phosphate via a common oxygen atom. The borate phosphates have a higher ratio of cations to number of borates and phosphates, as compared to the borophosphates.
The borate bromides are mixed anion compounds that contain borate and bromide anions. They are in the borate halide family of compounds which also includes borate fluorides, borate chlorides, and borate iodides.
Selenide borates, officially known as borate selenides, are chemical mixed anion compounds that contain any kind of borate and selenide ions. They are distinct from selenoborates in which selenium atoms replace oxygen in borates. There are also analogous borate sulfides, with sulfur ions instead of selenium.
Sulfidostannates, or thiostannates are chemical compounds containing anions composed of tin linked with sulfur. They can be considered as stannates with sulfur substituting for oxygen. Related compounds include the thiosilicates, and thiogermanates, and by varying the chalcogen: selenostannates, and tellurostannates. Oxothiostannates have oxygen in addition to sulfur. Thiostannates can be classed as chalcogenidometalates, thiometallates, chalcogenidotetrelates, thiotetrelates, and chalcogenidostannates. Tin is almost always in the +4 oxidation state in thiostannates, although a couple of mixed sulfides in the +2 state are known,
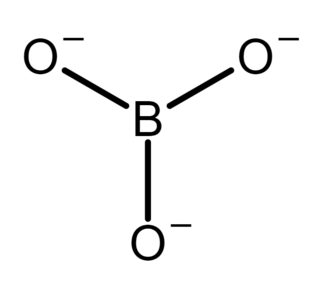
In inorganic chemistry, an orthoborate is a polyatomic anion with formula [BO3]3− or a salt containing the anion; such as trisodium orthoborate (Na+)3[BO3]3−. It is one of several boron oxyanions, or borates.
A fluorooxoiodate or fluoroiodate is a chemical compound or ion derived from iodate, by substituting some of the oxygen by fluorine. They have iodine in the +5 oxidation state. The iodine atoms have a stereochemically active lone-pair of electrons. Many are non-centrosymmetric, and are second harmonic generators (SHG) of intense light shining through them. They are under investigation as materials for non-linear optics, such as for generating ultraviolet light from visible or infrared lasers.
When values of birefingence are very high, the property is termed giant birefringence which more generically is called giant optical anisotropy. Values for giant birefringence exceed 0.3. Much bigger numbers are termed "colossal birefringence". These are achieved using nanostructures.
References
- 1 2 3 4 5 6 7 8 9 10 Zhang, Xueyan; Wu, Hongping; Yu, Hongwei; Yang, Zhihua; Pan, Shilie (2019-07-01). "Ba4M(CO3)2(BO3)2 (M=Ba, Sr): two borate-carbonates synthesized by open high temperature solution method". Science China Materials. 62 (7): 1023–1032. doi: 10.1007/s40843-018-9390-2 . ISSN 2199-4501. S2CID 139665355.
- ↑ Moureau, Magdeleine; Brace, Gerald. Dictionnaire des Science... (in French). Editions OPHRYS. p. 774. ISBN 978-2-7108-1109-1.
- ↑ Ferraris, G.; Franchini-Angela, M. (1 February 1978). "Canavesite, a new carboborate mineral from Brosso, Italy". The Canadian Mineralogist. 16 (1): 69–73.
- ↑ Douvris, Christos; Michl, Josef (9 October 2013). "Update 1 of: Chemistry of the Carba- closo -dodecaborate(−) Anion, CB11H12–". Chemical Reviews. 113 (10): PR179–PR233. doi:10.1021/cr400059k. PMID 23944158.
- ↑ Tanaka, Naoki; Shoji, Yoshiaki; Fukushima, Takanori (13 June 2016). "Convenient Route to Monocarba- closo -dodecaborate Anions". Organometallics. 35 (11): 2022–2025. doi:10.1021/acs.organomet.6b00309.
- ↑ "Qilianshanite". www.mindat.org. Retrieved 2020-12-14.
- ↑ "Canavesite". www.mindat.org. Retrieved 2020-12-14.
- ↑ Tombul, Mustafa; TüRkmenoglu, Elmas; Sahin, Onur (2021-08-10). "Unprecedented Formation of Potassium Borate Based Carbonate from Chloral Hydrate, Potassium Carbonate and Boric Acid". X-ray Structure Analysis Online. 37: 45–47. doi: 10.2116/xraystruct.37.45 . ISSN 1883-3578.
- ↑ Cao, Yuchen; Zhang, Bingbing; Liu, Hongkun; Li, Danni; Wang, Ying (2020). "NaK 15 [B 4 O 5 (OH) 4 ] 6 (NO 2 ) 2 (CO 3 )·7H 2 O: assembly of an unprecedented mixed anion inorganic compound via a facile hydrothermal route". New Journal of Chemistry. 44 (11): 4253–4256. doi:10.1039/D0NJ00249F. ISSN 1144-0546. S2CID 212875666.
- 1 2 3 4 5 Liu, Lili; Yang, Yun; Huang, Junben; Dong, Xiaoyu; Yang, Zhihua; Pan, Shilie (August 2017). "Design and Synthesis of a Series of Novel Mixed Borate and Carbonate Halides". Chemistry – A European Journal. 23 (43): 10451–10459. doi:10.1002/chem.201701926. ISSN 0947-6539. PMID 28575533.
- 1 2 Zhang, Xueyan; Wu, Hongping; Cheng, Shichao; Han, Guopeng; Yang, Zhihua; Pan, Shilie (2019-05-20). "K 9 [B 4 O 5 (OH) 4 ] 3 (CO 3 )X·7H 2 O (X = Cl, Br): Syntheses, Characterizations, and Theoretical Studies of Noncentrosymmetric Halogen Borate–Carbonates with Short UV Cutoff Edges". Inorganic Chemistry. 58 (10): 6974–6982. doi:10.1021/acs.inorgchem.9b00593. ISSN 0020-1669. PMID 31042371. S2CID 143422681.
- ↑ Yamnova, N. A.; Egorov-Tismenko, Yu. K.; Dimitrova, O. V.; Kantor, A. P. (2002-07-01). "Crystal structure of new synthetic Ca,Na,Li-carbonate-borate". Crystallography Reports. 47 (4): 566–573. Bibcode:2002CryRp..47..566Y. doi:10.1134/1.1496054. ISSN 1562-689X. S2CID 54984045.
- ↑ "Chiyokoite". www.mindat.org. Retrieved 2020-12-15.
- ↑ "Carboborite". www.mindat.org. Retrieved 2020-12-14.
- ↑ "Borcarite". www.mindat.org. Retrieved 2020-12-14.
- ↑ "Sakhaite". www.mindat.org. Retrieved 2020-12-14.
- ↑ "Harkerite". www.mindat.org. Retrieved 2020-12-14.
- ↑ "Imayoshiite". www.mindat.org. Retrieved 2020-12-15.
- ↑ "Gaudefroyite". www.mindat.org. Retrieved 2020-12-14.
- ↑ "Numanoite". www.mindat.org. Retrieved 2020-12-14.
- ↑ Ding, Fenghua; Zhang, Weilong; Nisbet, Matthew L.; Zhang, Weiguo; Halasyamani, P. Shiv; Yang, Zhihua; Pan, Shilie; Poeppelmeier, Kenneth R. (17 December 2019). "NaRb3B6O9(OH)3(HCO3): A Borate-Bicarbonate Nonlinear Optical Material". Inorganic Chemistry. 59 (1): 759–766. doi:10.1021/acs.inorgchem.9b03026. PMID 31846311. S2CID 209408459.
- ↑ Chen, Jiongquan; Yang, Zhihua; Wu, Kui; Yang, Yun; Pan, Shilie (January 2022). "Sr5(CO3)2(BO3)2: A new family member of isostructural mixed borate and carbonate Ba4M(BO3)2(CO3)2 (M = Ba, Sr) with isolated BO3 and CO3 groups". Journal of Molecular Structure. 1247: 131382. doi:10.1016/j.molstruc.2021.131382. S2CID 239659311.
- ↑ Belokoneva, E. L.; Dimitrova, O. V.; Mochenova, N. N. (January 2009). "Synthetic Na,Sr-carbonatoborate with a new type of pentaborate layer: The OD nature of the structure and its correlation with volkovskite, veatchites, and hilgardites". Crystallography Reports. 54 (1): 6–12. Bibcode:2009CryRp..54....6B. doi:10.1134/S1063774509010027. ISSN 1063-7745. S2CID 97956754.
- ↑ "Moydite-(Y)". www.mindat.org. Retrieved 2020-12-14.
- 1 2 Chen, Wei-Feng; Zou, Mei-Jun; Li, Jing-Jing; Zhang, Yi-Nan; Lan, You-Zhao; Cheng, Jian-Wen; Yang, Guo-Yu (2024-05-09). "M 6 [Cd 2 (CO 3 ) 2 (B 12 O 18 )(OH) 6 ] (M = K, Rb): Borate–Carbonates with Two CdCO 3 Embedded in a Cyclic Oxoboron Anion". Inorganic Chemistry. doi:10.1021/acs.inorgchem.4c01367. ISSN 0020-1669. PMID 38723292.
- 1 2 Liao, Chun-Jui; Wen, Yuh-Sheng; Lii, Kwang-Hwa (2018-09-17). "High-Temperature, High-Pressure Hydrothermal Synthesis of Ba 3 [B 6 O 10 (OH) 2 ](CO 3 ) and Ba 6 [B 12 O 21 (OH) 2 ](CO 3 ) 2 , Two Barium Borate Carbonates with 2D Layer and 3D Framework Structures". Inorganic Chemistry. 57 (18): 11492–11497. doi:10.1021/acs.inorgchem.8b01362. ISSN 0020-1669. PMID 30148619. S2CID 52094773.
- ↑ Heyward, Carla; McMillen, Colin D.; Kolis, Joseph (July 2013). "Hydrothermal synthesis and structural analysis of new mixed oxyanion borates: Ba11B26O44(PO4)2(OH)6, Li9BaB15O27(CO3) and Ba3Si2B6O16". Journal of Solid State Chemistry. 203: 166–173. Bibcode:2013JSSCh.203..166H. doi:10.1016/j.jssc.2013.04.022.
- ↑ Huang, Chunmei; Zhang, Fangfang; Cheng, Shichao; Yang, Zhihua; Li, Hao; Pan, Shilie (2020-11-16). "Ba 3 (BO 3 )(CO 3 )F: The First Borate Carbonate Fluoride Synthesized by the High-Temperature Solution Method". Chemistry – A European Journal. 26 (70): 16628–16632. doi:10.1002/chem.202003606. ISSN 0947-6539. PMID 32910472. S2CID 221620474.
- ↑ Abudoureheman, Maierhaba; Wang, Li; Zhang, Xianming; Yu, Hongwei; Yang, Zhihua; Lei, Chen; Han, Jian; Pan, Shilie (2015-04-20). "Pb 7 O(OH) 3 (CO 3 ) 3 (BO 3 ): First Mixed Borate and Carbonate Nonlinear Optical Material Exhibiting Large Second-Harmonic Generation Response". Inorganic Chemistry. 54 (8): 4138–4142. doi:10.1021/acs.inorgchem.5b00401. ISSN 0020-1669. PMID 25825990.
- ↑ Krivovichev, S. V.; Turner, R.; RumseY, M.; Siidra, O. I.; Kirk, C. A. (February 2009). "The crystal structure and chemistry of mereheadite". Mineralogical Magazine. 73 (1): 103–117. Bibcode:2009MinM...73..103K. doi:10.1180/minmag.2009.073.1.103. ISSN 0026-461X. S2CID 96947337.
- ↑ "Britvinite". www.mindat.org. Retrieved 2020-12-15.
- ↑ "Roymillerite". www.mindat.org. Retrieved 2020-12-15.