Related Research Articles
A solubility chart is a chart describing whether the ionic compounds formed from different combinations of cations and anions dissolve in or precipitate from solution.

The uranyl ion is an oxycation of uranium in the oxidation state +6, with the chemical formula UO2+
2. It has a linear structure with short U–O bonds, indicative of the presence of multiple bonds between uranium and oxygen. Four or more ligands may be bound to the uranyl ion in an equatorial plane around the uranium atom. The uranyl ion forms many complexes, particularly with ligands that have oxygen donor atoms. Complexes of the uranyl ion are important in the extraction of uranium from its ores and in nuclear fuel reprocessing.

A double salt is a salt that contains two or more different cations or anions. Examples of double salts include alums (with the general formula MIMIII(SO4)2·12H2O) and Tutton's salts (with the general formula (MI)2MII(SO4)2·6H2O). Other examples include potassium sodium tartrate, ammonium iron(II) sulfate (Mohr's salt), potassium uranyl sulfate (used to discover radioactivity) and bromlite BaCa(CO3)2. The fluorocarbonates contain fluoride and carbonate anions. Many coordination complexes form double salts.

Potassium ferrioxalate, also called potassium trisoxalatoferrate or potassium tris(oxalato)ferrate(III) is a chemical compound with the formula K3[Fe(C2O4)3]. It often occurs as the trihydrate K3[Fe(C2O4)3]·3H2O. Both are crystalline compounds, lime green in colour.

Manganese(II) oxide is an inorganic compound with chemical formula MnO. It forms green crystals. The compound is produced on a large scale as a component of fertilizers and food additives.

Magnesium oxalate is an organic compound comprising a magnesium cation with a 2+ charge bonded to an oxalate anion. It has the chemical formula MgC2O4. Magnesium oxalate is a white solid that comes in two forms: an anhydrous form and a dihydrate form where two water molecules are complexed with the structure. Both forms are practically insoluble in water and are insoluble in organic solutions.
The oxalatonickelates are a class of compounds that contain nickel complexed by oxalate groups. They form a series of double salts, and include clusters with multiple nickel atoms. Since oxalate functions as a bidentate ligand it can satisfy two coordinate positions around the nickel atom, or it can bridge two nickel atoms together.

Caesium oxalate (standard IUPAC spelling) dicesium oxalate, or cesium oxalate (American spelling) is the oxalate of caesium. Caesium oxalate has the chemical formula of Cs2C2O4.

A carbonate fluoride, fluoride carbonate, fluorocarbonate or fluocarbonate is a double salt containing both carbonate and fluoride. The salts are usually insoluble in water, and can have more than one kind of metal cation to make more complex compounds. Rare-earth fluorocarbonates are particularly important as ore minerals for the light rare-earth elements lanthanum, cerium and neodymium. Bastnäsite is the most important source of these elements. Other artificial compounds are under investigation as non-linear optical materials and for transparency in the ultraviolet, with effects over a dozen times greater than Potassium dideuterium phosphate.
The carbonate chlorides are double salts containing both carbonate and chloride anions. Quite a few minerals are known. Several artificial compounds have been made. Some complexes have both carbonate and chloride ligands. They are part of the family of halocarbonates. In turn these halocarbonates are a part of mixed anion materials.
The borate carbonates are mixed anion compounds containing both borate and carbonate ions. Compared to mixed anion compounds containing halides, these are quite rare. They are hard to make, requiring higher temperatures, which are likely to decompose carbonate to carbon dioxide. The reason for the difficulty of formation is that when entering a crystal lattice, the anions have to be correctly located, and correctly oriented. They are also known as borocarbonates. Although these compounds have been termed carboborate, that word also refers to the C=B=C5− anion, or CB11H12− anion. This last anion should be called 1-carba-closo-dodecaborate or monocarba-closo-dodecaborate.
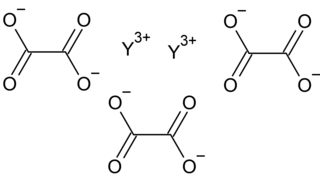
Yttrium oxalate is an inorganic compound, a salt of yttrium and oxalic acid with the chemical formula Y2(C2O4)3. The compound does not dissolve in water and forms crystalline hydrates—colorless crystals.

The oxalate phosphates are chemical compounds containing oxalate and phosphate anions. They are also called oxalatophosphates or phosphate oxalates. Some oxalate-phosphate minerals found in bat guano deposits are known. Oxalate phosphates can form metal organic framework compounds.
Manganese oxalate is a chemical compound, a salt of manganese and oxalic acid with the chemical formula MnC
2O
4. The compound creates light pink crystals, does not dissolve in water, and forms crystalline hydrates. It occurs naturally as the mineral Lindbergite.

Neodymium acetate is an inorganic salt composed of a neodymium atom trication and three acetate groups as anions where neodymium exhibits the +3 oxidation state. It has a chemical formula of Nd(CH3COO)3 although it can be informally referred to as NdAc because Ac is an informal symbol for acetate. It commonly occurs as a light purple powder.

Niobium(V) oxalate is the hydrogen oxalate salt of niobium(V). The neutral salt has not been prepared.
Oxalate sulfates are mixed anion compounds containing oxalate and sulfate. They are mostly transparent, and any colour comes from the cations.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.

Rubidium oxalate is the oxalate salt of rubidium, with the chemical formula of Rb2C2O4.
Sodium tris(carbonato)cobalt(III) is the name given to the inorganic compound with the formula Na3Co(CO3)3•3H2O. The salt contains an olive-green metastable cobalt(III) coordination complex. The salt is sometimes referred to as the “Field-Durrant precursor” and is prepared by the “Field-Durrant synthesis”. It is used in the synthesis of other cobalt(III) complexes. Otherwise cobalt(III) complexes are generated from cobalt(II) precursors, a process that requires an oxidant.
References
- ↑ Cindrić, Marina; Strukan, Neven; Vrdoljak, Višnja; Devčić, Maja; Kamenar, Boris (2002-01-01). "Synthesis of Molybdovanadates Coordinated by Oxalato Ligands. The Crystal Structure of K 6 [Mo 6 V 2 O 24 (C 2 O 4 ) 2 ]·6H 2 O". Journal of Coordination Chemistry. 55 (6): 705–710. doi:10.1080/00958970290027570. ISSN 0095-8972. S2CID 93516054.
- 1 2 Ma, Lin; Zhou, Xiaoliang; Yan, Zhengguang; Han, Xiaodong (May 2015). "Hydrothermal synthesis of [Y(H2O) ]2(C2O4)(CO3)2 powder using ascorbic acid". Advanced Powder Technology. 26 (3): 853–856. doi:10.1016/j.apt.2015.02.015.
- ↑ Fedorov, I. A.; Balakaeva, T. A. (1965-05-01). "SCANDIUM OXALATE-CARBONATE COMPOUNDS". Zh. Neorgan. Khim. (in Russian). 10. OSTI 4589394.
- ↑ Muraleedharan, K.; Labeeb, P.; Mujeeb, V. M. Abdul; Aneesh, M. H.; Devi, T. Ganga; Kannan, M. P. (2011-02-01). "Effect of Particle Size on Non-Isothermal Decomposition of Potassium Titanium Oxalate". Zeitschrift für Physikalische Chemie. 225 (2): 169–181. doi:10.1524/zpch.2011.0051. ISSN 2196-7156. S2CID 101959591.
- ↑ Matsuura, Tatsuo; Hashimoto, Tetsuo (December 1966). "Szilard-Chalmers Reaction of Anionic Cobalt Complex Ions on Ion-Exchange Resins". Bulletin of the Chemical Society of Japan. 39 (12): 2647–2652. doi: 10.1246/bcsj.39.2647 . ISSN 0009-2673.
- ↑ Enomoto, Yoshihiro; Ito, Teiichi; Shibata, Muraji (1974-05-05). "The Preparation Ofcis,Cis-Dicyanocarbonatodiamminecobalte(III) Andcis,Cis-Oxalatodiaquodiamminecobalt(III) Complexes". Chemistry Letters. 3 (5): 423–426. doi: 10.1246/cl.1974.423 . ISSN 0366-7022.
- ↑ Etape, Ekane Peter; Foba-Tendo, Josepha; Namondo, Beckley Victorine; Yufanyi, Divine Mbom; Tedjieukeng, Hypolite Mathias Kamta; Fomekonga, Roussin Lontio; Ngolui, Lambi John (2020-10-08). "Nanosize CaCu 3 Ti 4 O 12 Green Synthesis and Characterization of a Precursor Oxalate Obtained from Averrhoa carambola Fruit Juice and Its Thermal Decomposition to the Perovskite". Journal of Nanomaterials. 2020: 1–7. doi: 10.1155/2020/8830136 . ISSN 1687-4110.
- ↑ Bataille, Thierry; Louër, Daniel (2000-12-01). "Powder and single-crystal X-ray diffraction study of the structure of [Y(H 2 O)] 2 (C 2 O 4 )(CO 3 ) 2". Acta Crystallographica Section B: Structural Science. 56 (6): 998–1002. doi:10.1107/S0108768100010004. PMID 11099966.
- ↑ ВМ Погибко, ВВ Приседский, ИЛ Сидак (2010). "МЕХАНИЗМ ТЕРМИЧЕСКОГО РАЗЛОЖЕНИЯ ОКСАЛАТНОГО ПРЕКУРСОРА BaZrO3" (PDF). Archived (PDF) from the original on 2021-07-28.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ↑ Runde, Wolfgang (2009). "Directed Synthesis of Crystalline Plutonium(III) and (IV) Oxalates: Accessing Redox-Controlled Separations in Acidic Solutions". Inorganic Chemistry. 48 (13): 5967–5972. doi:10.1021/ic900344u. PMID 19485387. S2CID 13498583.
- 1 2 3 Romero, S.; Mosset, A.; Trombe, J.C. (December 1996). "Two New Families of Lanthanide Mixed-Ligand Complexes, Oxalate–Carbonate and Oxalate–Formate: Synthesis and Structure of [Ce(H2O)]2(C2O4)2(CO3)·2.5 H2O and Ce(C2O4)(HCO2)". Journal of Solid State Chemistry. 127 (2): 256–266. Bibcode:1996JSSCh.127..256R. doi:10.1006/jssc.1996.0382.
- ↑ Glasner, A.; Steinberg, M. (December 1961). "Thermal decomposition of the light rare earth oxalates". Journal of Inorganic and Nuclear Chemistry. 22 (1–2): 39–48. doi:10.1016/0022-1902(61)80227-3.
- ↑ Trombe, Jean-Christian; Galy, Jean; Enjalbert, Renée (2002-10-15). "A neodymium(III)–ammonium complex involving oxalate and carbonate ligands: (NH 4 ) 2 [Nd 2 (C 2 O 4 ) 3 (CO 3 )(H 2 O)]·H 2 O". Acta Crystallographica Section C: Crystal Structure Communications. 58 (10): m517–m520. doi:10.1107/S0108270102014737. ISSN 0108-2701. PMID 12359932.
- 1 2 3 4 5 Romero, S (1997). "A new family of lanthanide oxalate carbonate, [Ln(H2O)]2(C2O4)(CO3)2 with Ln = Eu...Ho, presenting a relatively open framework". European Journal of Solid State and Inorganic Chemistry. 34: 209–219.
- ↑ Müller-Buschbaum, Klaus (2002). "Er2(CO3)2(C2O4)(H2O)2 — Synthese, Kristallstruktur und thermischer Abbau eines Carbonat-Oxalat-Hydrates des Erbiums". Zeitschrift für anorganische und allgemeine Chemie (in German). 628 (8): 1761–1764. doi:10.1002/1521-3749(200208)628:8<1761::AID-ZAAC1761>3.0.CO;2-1. ISSN 1521-3749.
- ↑ Yang, Hui; Mao, Feifei; Xu, Cuixia (2018-05-24). "Syntheses, Crystal Structures and Properties of Two Novel Mixed Anion Hybrids with Formulas of Pb 6 O 2 (BO 3 ) 2 (C 2 O 4 ) and Pb 4 (CO 3 ) 2 (C 2 O 4 )(OH) 2". ChemistrySelect. 3 (19): 5431–5438. doi:10.1002/slct.201801072. ISSN 2365-6549.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 Bagnall, Kenneth W. (2013-12-12). Th Thorium: Compounds with Carbon: Carbonates, Thiocyanates, Alkoxides, Carboxylates. Springer Science & Business Media. pp. 98–102. ISBN 978-3-662-06315-6.
- ↑ Bagnall, Kenneth W. (2013-12-12). Th Thorium: Compounds with Carbon: Carbonates, Thiocyanates, Alkoxides, Carboxylates. Springer Science & Business Media. p. 10. ISBN 978-3-662-06315-6.
- ↑ L. N. Essen, D. P. Alekseeva. "The production of mixed oxalate-carbonate complex compounds of thorium", Dokl. Akad. Nauk SSSR, 146:2 (1962), 380–382". www.mathnet.ru. Archived from the original on 2021-07-27. Retrieved 2021-07-27.
- 1 2 3 4 5 Beirakhov, A. G.; Orlova, I. M.; Mikhailov, Yu. N. (December 2014). "Coordination modes of α-dioximes in uranyl complexes". Russian Journal of Inorganic Chemistry. 59 (14): 1679–1690. doi:10.1134/S0036023614140022. ISSN 0036-0236. S2CID 95903987.
- ↑ Beirakhov, A. G.; Orlova, I. M.; Il’in, E. G.; Aleksandrov, G. G.; Kanishcheva, A. S.; Mikhailov, Yu. N. (September 2010). "Uranyl complexes with ethylmethylglyoxime". Russian Journal of Inorganic Chemistry. 55 (9): 1373–1380. doi:10.1134/S003602361009007X. ISSN 0036-0236. S2CID 97458234.
- 1 2 Beirakhov, A. G.; Orlova, I. M.; Il’in, E. G.; Kanishcheva, A. S.; Churakov, A. V.; Mikhailov, Yu. N. (July 2012). "Uranyl complexes with methyl derivatives of alicyclic dioximes". Russian Journal of Inorganic Chemistry. 57 (7): 945–952. doi:10.1134/S0036023612070054. ISSN 0036-0236. S2CID 97320941.
- 1 2 Beirakhov, A. G.; Orlova, I. M.; Rotov, A. V.; Il’in, E. G.; Goeva, L. V.; Surazhskaya, M. D.; Churakov, A. V.; Mikhailov, Yu. N. (December 2016). "Conformation of diethylglyoxime in uranyl complexes". Russian Journal of Inorganic Chemistry. 61 (12): 1522–1529. doi:10.1134/S0036023616120032. ISSN 0036-0236. S2CID 100478539.
- ↑ Fedoseev, A. M.; Grigor’ev, M. S.; Yusov, A. B. (September 2012). "Interaction of tetravalent actinides and Np(V) with some hydroxy carboxylic acids". Radiochemistry. 54 (5): 443–451. doi:10.1134/S1066362212050049. ISSN 1066-3622. S2CID 95387998.
- ↑ Kartushova, R. E.; Rudenko, T. I.; Fomin, V. V. (1958). "Thermal decomposition of tetravalent and trivalent plutonium oxalates" (PDF). The Soviet Journal of Atomic Energy. 5 (1): 831–835. doi:10.1007/BF01505392. S2CID 94605282.