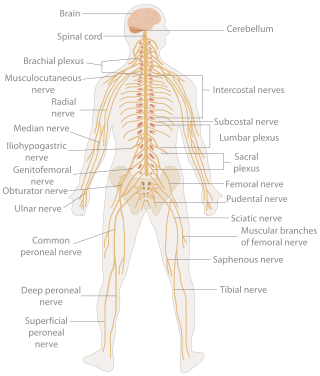
In biology, the nervous system is the highly complex part of an animal that coordinates its actions and sensory information by transmitting signals to and from different parts of its body. The nervous system detects environmental changes that impact the body, then works in tandem with the endocrine system to respond to such events. Nervous tissue first arose in wormlike organisms about 550 to 600 million years ago. In vertebrates, it consists of two main parts, the central nervous system (CNS) and the peripheral nervous system (PNS). The CNS consists of the brain and spinal cord. The PNS consists mainly of nerves, which are enclosed bundles of the long fibers, or axons, that connect the CNS to every other part of the body. Nerves that transmit signals from the brain are called motor nerves (efferent), while those nerves that transmit information from the body to the CNS are called sensory nerves (afferent). The PNS is divided into two separate subsystems, the somatic and autonomic, nervous systems. The autonomic nervous system is further subdivided into the sympathetic, parasympathetic and enteric nervous systems. The sympathetic nervous system is activated in cases of emergencies to mobilize energy, while the parasympathetic nervous system is activated when organisms are in a relaxed state. The enteric nervous system functions to control the gastrointestinal system. Nerves that exit from the brain are called cranial nerves while those exiting from the spinal cord are called spinal nerves.

Behavioral neuroscience, also known as biological psychology, biopsychology, or psychobiology, is the application of the principles of biology to the study of physiological, genetic, and developmental mechanisms of behavior in humans and other animals.

In genetic engineering, a gene gun or biolistic particle delivery system is a device used to deliver exogenous DNA (transgenes), RNA, or protein to cells. By coating particles of a heavy metal with a gene of interest and firing these micro-projectiles into cells using mechanical force, an integration of desired genetic information can be introduced into desired cells. The technique involved with such micro-projectile delivery of DNA is often referred to as biolistics, short for "biological ballistics".
Cre-Lox recombination is a site-specific recombinase technology, used to carry out deletions, insertions, translocations and inversions at specific sites in the DNA of cells. It allows the DNA modification to be targeted to a specific cell type or be triggered by a specific external stimulus. It is implemented both in eukaryotic and prokaryotic systems. The Cre-lox recombination system has been particularly useful to help neuroscientists to study the brain in which complex cell types and neural circuits come together to generate cognition and behaviors. NIH Blueprint for Neuroscience Research has created several hundreds of Cre driver mouse lines which are currently used by the worldwide neuroscience community.

In genetics, Flp-FRT recombination is a site-directed recombination technology, increasingly used to manipulate an organism's DNA under controlled conditions in vivo. It is analogous to Cre-lox recombination but involves the recombination of sequences between short flippase recognition target (FRT) sites by the recombinase flippase (Flp) derived from the 2 μ plasmid of baker's yeast Saccharomyces cerevisiae.
Optogenetics is a biological technique to control the activity of neurons or other cell types with light. This is achieved by expression of light-sensitive ion channels, pumps or enzymes specifically in the target cells. On the level of individual cells, light-activated enzymes and transcription factors allow precise control of biochemical signaling pathways. In systems neuroscience, the ability to control the activity of a genetically defined set of neurons has been used to understand their contribution to decision making, learning, fear memory, mating, addiction, feeding, and locomotion. In a first medical application of optogenetic technology, vision was partially restored in a blind patient with Retinitis pigmentosa.
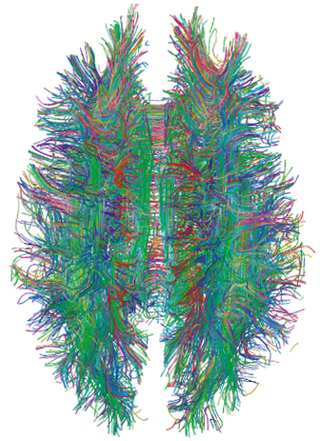
A connectome is a comprehensive map of neural connections in the brain, and may be thought of as its "wiring diagram". An organism's nervous system is made up of neurons which communicate through synapses. A connectome is constructed by tracing the neuron in a nervous system and mapping where neurons are connected through synapses.
Connectomics is the production and study of connectomes: comprehensive maps of connections within an organism's nervous system. More generally, it can be thought of as the study of neuronal wiring diagrams with a focus on how structural connectivity, individual synapses, cellular morphology, and cellular ultrastructure contribute to the make up of a network. The nervous system is a network made of billions of connections and these connections are responsible for our thoughts, emotions, actions, memories, function and dysfunction. Therefore, the study of connectomics aims to advance our understanding of mental health and cognition by understanding how cells in the nervous system are connected and communicate. Because these structures are extremely complex, methods within this field use a high-throughput application of functional and structural neural imaging, most commonly magnetic resonance imaging (MRI), electron microscopy, and histological techniques in order to increase the speed, efficiency, and resolution of these nervous system maps. To date, tens of large scale datasets have been collected spanning the nervous system including the various areas of cortex, cerebellum, the retina, the peripheral nervous system and neuromuscular junctions.

Retrograde tracing is a research method used in neuroscience to trace neural connections from their point of termination to their source. Retrograde tracing techniques allow for detailed assessment of neuronal connections between a target population of neurons and their inputs throughout the nervous system. These techniques allow the "mapping" of connections between neurons in a particular structure and the target neurons in the brain. The opposite technique is anterograde tracing, which is used to trace neural connections from their source to their point of termination. Both the anterograde and retrograde tracing techniques are based on the visualization of axonal transport.
Guidepost cells are cells which assist in the subcellular organization of both neural axon growth and migration. They act as intermediate targets for long and complex axonal growths by creating short and easy pathways, leading axon growth cones towards their target area.

In genetics, floxing refers to the sandwiching of a DNA sequence between two lox P sites. The terms are constructed upon the phrase "flanking/flanked by LoxP". Recombination between LoxP sites is catalysed by Cre recombinase. Floxing a gene allows it to be deleted, translocated or inverted in a process called Cre-Lox recombination. The floxing of genes is essential in the development of scientific model systems as it allows researchers to have spatial and temporal alteration of gene expression. Moreover, animals such as mice can be used as models to study human disease. Therefore, Cre-lox system can be used in mice to manipulate gene expression in order to study human diseases and drug development. For example, using the Cre-lox system, researchers can study oncogenes and tumor suppressor genes and their role in development and progression of cancer in mice models.

GCaMP is a genetically encoded calcium indicator (GECI) initially developed in 2001 by Junichi Nakai. It is a synthetic fusion of green fluorescent protein (GFP), calmodulin (CaM), and M13, a peptide sequence from myosin light-chain kinase. When bound to Ca2+, GCaMP fluoresces green with a peak excitation wavelength of 480 nm and a peak emission wavelength of 510 nm. It is used in biological research to measure intracellular Ca2+ levels both in vitro and in vivo using virally transfected or transgenic cell and animal lines. The genetic sequence encoding GCaMP can be inserted under the control of promoters exclusive to certain cell types, allowing for cell-type specific expression of GCaMP. Since Ca2+ is a second messenger that contributes to many cellular mechanisms and signaling pathways, GCaMP allows researchers to quantify the activity of Ca2+-based mechanisms and study the role of Ca2+ ions in biological processes of interest.
Tamily Weissman-Unni is a neurobiology professor at Lewis and Clark College in Portland Oregon. She has published numerous studies on brain development and her current research focuses on formation of cerebellar circuits. Additionally, she has won several scientific imaging competitions for her striking multi-color pictures of the brain.
Fasciclin 2 is a 95 kilodalton cell membrane glycoprotein in the immunoglobulin (Ig) – related superfamily of cell adhesion molecules (CAMs). It was first identified in the developing grasshopper embryo, seen dynamically expressed on a subset of fasciculating axons in the central nervous system (CNS), functioning as a neuronal recognition molecule in the regulation of selective axon fasciculation. Subsequently, fasII was cloned and has mainly been studied in the fruit fly. Its extracellular structure consists of two Fibronectin type III domains and five Ig-like C2 domains, having structural homology to the neural cell adhesion molecule (NCAM) found in vertebrates. Alternative splicing of fasII gives rise to its expression in three major isoforms, including a membrane-associated form that is attached to the outer leaflet of the plasma membrane via a glycophosphatidylinositol linkage and two integral transmembrane forms. The larger transmembrane form has an amino acid motif contained in its cytoplasmic domain that is rich in proline, glutamic acid, serine and threonine residues. The fasciclin 1 (Fas1) and fasciclin 3 (Fas3) genes in Drosophila also code for cell adhesion proteins in the nervous system but do not show any structural or functional similarities with NCAM.

A neuronal lineage marker is an endogenous tag that is expressed in different cells along neurogenesis and differentiated cells such as neurons. It allows detection and identification of cells by using different techniques. A neuronal lineage marker can be either DNA, mRNA or RNA expressed in a cell of interest. It can also be a protein tag, as a partial protein, a protein or an epitope that discriminates between different cell types or different states of a common cell. An ideal marker is specific to a given cell type in normal conditions and/or during injury. Cell markers are very valuable tools for examining the function of cells in normal conditions as well as during disease. The discovery of various proteins specific to certain cells led to the production of cell-type-specific antibodies that have been used to identify cells.

Neuronal self-avoidance, or isoneural avoidance, is an important property of neurons which consists in the tendency of branches arising from a single soma to turn away from one another. The arrangements of branches within neuronal arbors are established during development and result in minimal crossing or overlap as they spread over a territory, resulting in the typical fasciculated morphology of neurons.
Joe Z. Tsien(钱卓) is a neuroscientist who pioneered Cre/lox-neurogenetics in the mid-1990s, a versatile toolbox for neuroscientists to study the complex relationships between genes, neural circuits, and behaviors. He is also known as the creator of the smart mouse Doogie in the late 1990s while being a faculty member at Princeton University.
Jeff W. Lichtman is an American neuroscientist. He is the Jeremy R. Knowles Professor of Molecular and Cellular Biology and Santiago Ramón y Cajal Professor of Arts and Sciences at Harvard University. He is best known for his pioneering work developing the neuroimaging connectomic technique known as Brainbow.
Susan M. Dymecki is an American geneticist and neuroscientist and director of the Biological and Biomedical Sciences PhD Program at Harvard University. Dymecki is also a professor in the Department of Genetics and the principal investigator of the Dymecki Lab at Harvard. Her lab characterizes the development and function of unique populations of serotonergic neurons in the mouse brain. To enable this functional dissection, Dymecki has pioneered several transgenic tools for probing neural circuit development and function. Dymecki also competed internationally as an ice dancer, placing 7th in the 1980 U.S. Figure Skating Championships.

Genome editing of synthetic target arrays for lineage tracing (GESTALT) is a method used to determine the developmental lineages of cells in multicellular systems. GESTALT involves introducing a small DNA barcode that contains regularly spaced CRISPR/Cas9 target sites into the genomes of progenitor cells. Alongside the barcode, Cas9 and sgRNA are introduced into the cells. Mutations in the barcode accumulate during the course of cell divisions and the unique combination of mutations in a cell's barcode can be determined by DNA or RNA sequencing to link it to a developmental lineage.















