Violet is the color of light at the short wavelength end of the visible spectrum. It is one of the seven colors that Isaac Newton labeled when dividing the spectrum of visible light in 1672. Violet light has a wavelength between approximately 380 and 435 nanometers. The color's name is derived from the Viola genus of flowers.

Purple is a color similar in appearance to violet light. In the RYB color model historically used in the arts, purple is a secondary color created by combining red and blue pigments. In the CMYK color model used in modern printing, purple is made by combining magenta pigment with either cyan pigment, black pigment, or both. In the RGB color model used in computer and television screens, purple is created by mixing red and blue light in order to create colors that appear similar to violet light.

Brown is a color. It can be considered a composite color, but it is mainly a darker shade of orange. In the CMYK color model used in printing and painting, brown is usually made by combining the colors orange and black. In the RGB color model used to project colors onto television screens and computer monitors, brown combines red and green.

A pigment is a powder used to add color or change visual appearance. Pigments are completely or nearly insoluble and chemically unreactive in water or another medium; in contrast, dyes are colored substances which are soluble or go into solution at some stage in their use. Dyes are often organic compounds whereas pigments are often inorganic. Pigments of prehistoric and historic value include ochre, charcoal, and lapis lazuli.

Indigo dye is an organic compound with a distinctive blue color. Indigo is a natural dye extracted from the leaves of some plants of the Indigofera genus, in particular Indigofera tinctoria; dye-bearing Indigofera plants were commonly grown and used throughout the world, in Asia in particular, as an important crop, with the production of indigo dyestuff economically important due to the historical rarity of other blue dyestuffs.

Tyrian purple, also known as royal purple, imperial purple, or imperial dye, is a reddish-purple natural dye. The name Tyrian refers to Tyre, Lebanon. It is secreted by several species of predatory sea snails in the family Muricidae, rock snails originally known by the name Murex. In ancient times, extracting this dye involved tens of thousands of snails and substantial labour, and as a result, the dye was highly valued. The colored compound is 6,6'-dibromoindigo.
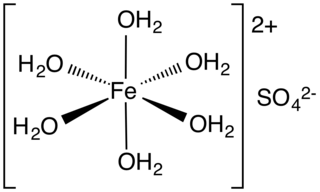
Iron(II) sulfate (British English: iron(II) sulphate) or ferrous sulfate denotes a range of salts with the formula Fe SO4·xH2O. These compounds exist most commonly as the heptahydrate (x = 7) but several values for x are known. The hydrated form is used medically to treat or prevent iron deficiency, and also for industrial applications. Known since ancient times as copperas and as green vitriol (vitriol is an archaic name for sulfate), the blue-green heptahydrate (hydrate with 7 molecules of water) is the most common form of this material. All the iron(II) sulfates dissolve in water to give the same aquo complex [Fe(H2O)6]2+, which has octahedral molecular geometry and is paramagnetic. The name copperas dates from times when the copper(II) sulfate was known as blue copperas, and perhaps in analogy, iron(II) and zinc sulfate were known respectively as green and white copperas.

Prussian blue is a dark blue pigment produced by oxidation of ferrous ferrocyanide salts. It has the chemical formula FeIII
4[FeII
(CN)
6]
3. Turnbull's blue is chemically identical, but is made from different reagents, and its slightly different color stems from different impurities and particle sizes.

Alizarin is an organic compound with formula C
14H
8O
4 that has been used throughout history as a prominent red dye, principally for dyeing textile fabrics. Historically it was derived from the roots of plants of the madder genus. In 1869, it became the first natural dye to be produced synthetically.
Carmine – also called cochineal, cochineal extract, crimson lake, or carmine lake – is a pigment of a bright-red color obtained from the aluminium complex derived from carminic acid. Specific code names for the pigment include natural red 4, C.I. 75470, or E120. Carmine is also a general term for a particularly deep-red color.

Umber is a natural earth pigment consisting of iron oxide and manganese oxide; it has a brownish color that can vary among shades of yellow, red, and green. Umber is considered one of the oldest pigments known to humans, first seen in Ajanta Caves in 200 BC – 600 AD. Umber's advantages are its highly versatile color, warm tone, and quick drying abilities. While some sources indicate that umber's name comes from its geographic origin in Umbria, other scholars suggest that it derives from the Latin word umbra, which means "shadow". The belief that its name derives from the word for shadow is fitting, as the color helps create shadows. The color is primarily produced in Cyprus. Umber is typically mined from open pits or underground mines and ground into a fine powder that is washed to remove impurities. In the 20th century, the rise of synthetic dyes decreased the demand for natural pigments such as umber.
Venetian red is a light and warm (somewhat unsaturated) pigment that is a darker shade of red. The composition of Venetian red changed over time. Originally it consisted of natural ferric oxide (Fe2O3, partially hydrated) obtained from the red hematite. Modern versions are frequently made with synthetic red iron oxide produced via calcination of green vitriol (a.k.a. copperas) mixed with white chalk. The pigment contains up to 50% of the ferric oxide.
Caput mortuum is a Latin term whose literal meaning is "dead head" or "worthless remains", used in alchemy.

Lead(II,IV) oxide, also called red lead or minium, is the inorganic compound with the formula Pb3O4. A bright red or orange solid, it is used as pigment, in the manufacture of batteries, and rustproof primer paints. It is an example of a mixed valence compound, being composed of both Pb(II) and Pb(IV) in the ratio of two to one.

Natural dyes are dyes or colorants derived from plants, invertebrates, or minerals. The majority of natural dyes are vegetable dyes from plant sources—roots, berries, bark, leaves, and wood—and other biological sources such as fungi.

Dyeing is the craft of imparting colors to textiles in loose fiber, yarn, cloth or garment form by treatment with a dye. Archaeologists have found evidence of textile dyeing with natural dyes dating back to the Neolithic period. In China, dyeing with plants, barks and insects has been traced back more than 5,000 years. Natural insect dyes such as Tyrian purple and kermes and plant-based dyes such as woad, indigo and madder were important elements of the economies of Asia and Europe until the discovery of man-made synthetic dyes in the mid-19th century. Synthetic dyes quickly superseded natural dyes for the large-scale commercial textile production enabled by the industrial revolution, but natural dyes remained in use by traditional cultures around the world.

A colorant is any substance that changes the spectral transmittance or reflectance of a material. Synthetic colorants are those created in a laboratory or industrial setting. The production and improvement of colorants was a driver of the early synthetic chemical industry, in fact many of today's largest chemical producers started as dye-works in the late 19th or early 20th centuries, including Bayer AG(1863). Synthetics are extremely attractive for industrial and aesthetic purposes as they have they often achieve higher intensity and color fastness than comparable natural pigments and dyes used since ancient times. Market viable large scale production of dyes occurred nearly simultaneously in the early major producing countries Britain (1857), France (1858), Germany (1858), and Switzerland (1859), and expansion of associated chemical industries followed. The mid-nineteenth century through WWII saw an incredible expansion of the variety and scale of manufacture of synthetic colorants. Synthetic colorants quickly became ubiquitous in everyday life, from clothing to food. This stems from the invention of industrial research and development laboratories in the 1870s, and the new awareness of empirical chemical formulas as targets for synthesis by academic chemists. The dye industry became one of the first instances where directed scientific research lead to new products, and the first where this occurred regularly.

Blue pigments are natural or synthetic materials, usually made from minerals and insoluble with water, used to make the blue colors in painting and other arts. The raw material of the earliest blue pigment was lapis lazuli from mines in Afghanistan, that was refined into the pigment ultramarine. Since the late 18th and 19th century, blue pigments are largely synthetic, manufactured in laboratories and factories.

Red pigments are materials, usually made from minerals, used to create the red colors in painting and other arts. The color of red and other pigments is determined by the way it absorbs certain parts of the spectrum of visible light and reflects the others. The brilliant opaque red of vermillion, for example, results because vermillion reflects the major part of red light, but absorbs the blue, green and yellow parts of white light.