Related Research Articles
Nerve agents, sometimes also called nerve gases, are a class of organic chemicals that disrupt the mechanisms by which nerves transfer messages to organs. The disruption is caused by the blocking of acetylcholinesterase (AChE), an enzyme that catalyzes the breakdown of acetylcholine, a neurotransmitter. Nerve agents are irreversible acetylcholinesterase inhibitors used as poison.

Atropine is a tropane alkaloid and anticholinergic medication used to treat certain types of nerve agent and pesticide poisonings as well as some types of slow heart rate, and to decrease saliva production during surgery. It is typically given intravenously or by injection into a muscle. Eye drops are also available which are used to treat uveitis and early amblyopia. The intravenous solution usually begins working within a minute and lasts half an hour to an hour. Large doses may be required to treat some poisonings.

VX is an extremely toxic synthetic chemical compound in the organophosphorus class, specifically, a thiophosphonate. In the class of nerve agents, it was developed for military use in chemical warfare after translation of earlier discoveries of organophosphate toxicity in pesticide research. In its pure form, VX is an oily, relatively non-volatile liquid that is amber-like in colour. Because of its low volatility, VX persists in environments where it is dispersed.
Anticholinergics are substances that block the action of the acetylcholine (ACh) neurotransmitter at synapses in the central and peripheral nervous system.

Chlorfenvinphos is an organophosphorus compound that was widely used as an insecticide and an acaricide. The molecule itself can be described as an enol ester derived from dichloroacetophenone and diethylphosphonic acid. Chlorfenvinphos has been included in many products since its first use in 1963. However, because of its toxic effect as a cholinesterase inhibitor it has been banned in several countries, including the United States and the European Union. Its use in the United States was cancelled in 1991.
Obidoxime is a member of the oxime family used to treat organophosphate poisoning. Oximes are drugs known for their ability to reverse the binding of organophosphorus compounds to the enzyme acetylcholinesterase (AChE).

Diazinon, a colorless to dark brown liquid, is a thiophosphoric acid ester developed in 1952 by Ciba-Geigy, a Swiss chemical company. It is a nonsystemic organophosphate insecticide formerly used to control cockroaches, silverfish, ants, and fleas in residential, non-food buildings. Diazinon was heavily used during the 1970s and early 1980s for general-purpose gardening use and indoor pest control. A bait form was used to control scavenger wasps in the western U.S. Diazinon is used in flea collars for domestic pets in Australia and New Zealand. Diazinon is a major component in the "Golden Fleece" brand sheep dip. Residential uses of diazinon were outlawed in the U.S. in 2004 because of human health risks but it is still approved for agricultural uses. An emergency antidote is atropine.
A cholinergic crisis is an over-stimulation at a neuromuscular junction due to an excess of acetylcholine (ACh), as a result of the inactivity of the AChE enzyme, which normally breaks down acetylcholine.

Pralidoxime or 2-PAM, usually as the chloride or iodide salts, belongs to a family of compounds called oximes that bind to organophosphate-inactivated acetylcholinesterase. It is used to treat organophosphate poisoning in conjunction with atropine and either diazepam or midazolam. It is a white solid.

Azinphos-methyl (Guthion) is a broad spectrum organophosphate insecticide manufactured by Bayer CropScience, Gowan Co., and Makhteshim Agan. Like other pesticides in this class, it owes its insecticidal properties to the fact that it is an acetylcholinesterase inhibitor. It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act, and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.

Phosmet is a phthalimide-derived, non-systemic, organophosphate insecticide used on plants and animals. It is mainly used on apple trees for control of codling moth, though it is also used on a wide range of fruit crops, ornamentals, and vines for the control of aphids, suckers, mites, and fruit flies.

Dioxathion, systematically known as p-dioxane-2,3-diyl ethyl phosphorodithioate, is an organophosphate pesticide. It is used as an insecticide on livestock and as an acaricide on citrus fruits, deciduous fruits and nuts.
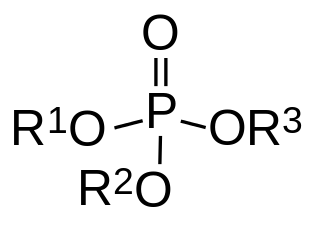
Organophosphate poisoning is poisoning due to organophosphates (OPs). Organophosphates are used as insecticides, medications, and nerve agents. Symptoms include increased saliva and tear production, diarrhea, vomiting, small pupils, sweating, muscle tremors, and confusion. While onset of symptoms is often within minutes to hours, some symptoms can take weeks to appear. Symptoms can last for days to weeks.
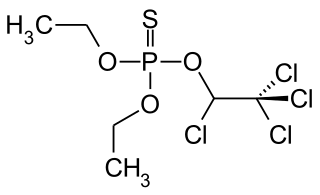
Chlorethoxyfos is an organophosphate acetylcholinesterase inhibitor used as an insecticide. It is registered for the control of corn rootworms, wireworms, cutworms, seed corn maggot, white grubs and symphylans on corn. The insecticide is sold under the trade name Fortress by E.I. du Pont de Nemours & Company.

Disulfoton is an organophosphate acetylcholinesterase inhibitor used as an insecticide. It is manufactured under the name Di-Syston by Bayer CropScience. Disulfoton in its pure form is a colorless oil but the technical product used in vegetable fields is dark and yellowish with a sulfur odor. Disulfoton is processed as a liquid into carrier granules, these granules are mixed with fertilizer and clay to be made into a spike, designed to be driven into the ground. The pesticide is absorbed over time by the roots and translocated to all parts of the plant. The pesticide acts as a cholinesterase inhibitor and gives long lasting control.

Cholinesterase inhibitors (ChEIs), also known as anti-cholinesterase, are chemicals that prevent the breakdown of the neurotransmitter acetylcholine or butyrylcholine. This increases the amount of the acetylcholine or butyrylcholine in the synaptic cleft that can bind to muscarinic receptors, nicotinic receptors and others. This group of inhibitors is divided into two subgroups, acetylcholinesterase inhibitors (AChEIs) and butyrylcholinesterase inhibitors (BChEIs).

Ethoprophos (or ethoprop) is an organophosphate ester with the formula C8H19O2PS2. It is a clear yellow to colourless liquid that has a characteristic mercaptan-like odour. It is used as an insecticide and nematicide and it is an acetylcholinesterase inhibitor.

Terbufos is a chemical compound used in insecticides and nematicides. It is part of the chemical family of organophosphates. It is a clear, colourless to pale yellow or reddish-brown liquid and sold commercially as granulate.

Triazofos is a chemical compound used in acaricides, insecticides, and nematicides.

Guanitoxin (GNT), formerly known as anatoxin-a(S) "Salivary", is a naturally occurring cyanotoxin commonly isolated from cyanobacteria. It is a potent covalent acetylcholinesterase inhibitor, and thus a potent rapid acting neurotoxin which in cases of severe exposure can lead to death. Guanitoxin was first structurally characterized in 1989, and consists of a cyclic N-hydroxyguanine organophosphate with a phosphate ester moiety.
References
- 1 2 3 4 5 6 7 8 9 10 Vohra, Rais (2022). "Organophosphorus and carbamate insecticides". Poisoning & Drug Overdose (8th ed.). McGraw Hill.
- 1 2 Jayawardane, Pradeepa; Senanayake, Nimal; Buckley, Nick A.; Dawson, Andrew H. (2012). "Electrophysiological correlates of respiratory failure in acute organophosphate poisoning: Evidence for differential roles of muscarinic and nicotinic stimulation". Clinical Toxicology. 50 (4): 250–253. doi:10.3109/15563650.2012.670875. ISSN 1556-3650. PMC 3357897 . PMID 22455356.
- 1 2 3 4 King, Andrew M.; Aaron, Cynthia K. (2015-02-01). "Organophosphate and Carbamate Poisoning". Emergency Medicine Clinics of North America. Management of Hazardous Material Emergencies. 33 (1): 133–151. doi:10.1016/j.emc.2014.09.010. ISSN 0733-8627. PMID 25455666.
- ↑ Eddleston, Michael; Buckley, Nick A; Eyer, Peter; Dawson, Andrew H (February 2008). "Management of acute organophosphorus pesticide poisoning". The Lancet. 371 (9612): 597–607. doi:10.1016/S0140-6736(07)61202-1. PMC 2493390 . PMID 17706760.
- ↑ Kumar, Sachil; Baggi, Tulsidas R.; Zughaibi, Torki (2022-11-01). "Forensic toxicological and analytical aspects of carbamate poisoning – A review". Journal of Forensic and Legal Medicine. 92: 102450. doi:10.1016/j.jflm.2022.102450. ISSN 1752-928X. PMID 36399917. S2CID 253433639.