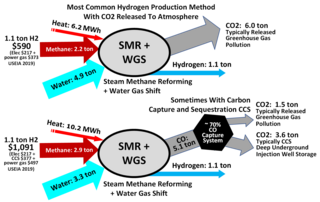
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly natural gas is the feedstock. The main purpose of this technology is hydrogen production. The reaction is represented by this equilibrium:

The methanol economy is a suggested future economy in which methanol and dimethyl ether replace fossil fuels as a means of energy storage, ground transportation fuel, and raw material for synthetic hydrocarbons and their products. It offers an alternative to the proposed hydrogen economy or ethanol economy, although these concepts are not exclusive. Methanol can be produced from a variety of sources including fossil fuels as well as agricultural products and municipal waste, wood and varied biomass. It can also be made from chemical recycling of carbon dioxide.

Synthetic fuel or synfuel is a liquid fuel, or sometimes gaseous fuel, obtained from syngas, a mixture of carbon monoxide and hydrogen, in which the syngas was derived from gasification of solid feedstocks such as coal or biomass or by reforming of natural gas.

Coal pollution mitigation, sometimes labeled as clean coal, is a series of systems and technologies that seek to mitigate health and environmental impact of burning coal for energy. Burning coal releases harmful substances, including mercury, lead, sulfur dioxide (SO2), nitrogen oxides (NOx), and carbon dioxide (CO2), contributing to air pollution, acid rain, and greenhouse gas emissions. Methods include flue-gas desulfurization, selective catalytic reduction, electrostatic precipitators, and fly ash reduction focusing on reducing the emissions of these harmful substances. These measures aim to reduce coal's impact on human health and the environment.

Biomass to liquid is a multi-step process of producing synthetic hydrocarbon fuels made from biomass via a thermochemical route.

Gas to liquids (GTL) is a refinery process to convert natural gas or other gaseous hydrocarbons into longer-chain hydrocarbons, such as gasoline or diesel fuel. Methane-rich gases are converted into liquid synthetic fuels. Two general strategies exist: (i) direct partial combustion of methane to methanol and (ii) Fischer–Tropsch-like processes that convert carbon monoxide and hydrogen into hydrocarbons. Strategy ii is followed by diverse methods to convert the hydrogen-carbon monoxide mixtures to liquids. Direct partial combustion has been demonstrated in nature but not replicated commercially. Technologies reliant on partial combustion have been commercialized mainly in regions where natural gas is inexpensive.

Carbon capture and storage (CCS) is a process in which a relatively pure stream of carbon dioxide (CO2) from industrial sources is separated, treated and transported to a long-term storage location. For example, the carbon dioxide stream that is to be captured can result from burning fossil fuels or biomass. Usually the CO2 is captured from large point sources, such as a chemical plant or biomass plant, and then stored in an underground geological formation. The aim is to reduce greenhouse gas emissions and thus mitigate climate change. The IPCC's most recent report on mitigating climate change describes CCS retrofits for existing power plants as one of the ways to limit emissions from the electricity sector and meet Paris Agreement goals.

Carbon sequestration is the process of storing carbon in a carbon pool. Carbon sequestration is a naturally occurring process but it can also be enhanced or achieved with technology, for example within carbon capture and storage projects. There are two main types of carbon sequestration: geologic and biologic.
An integrated gasification combined cycle (IGCC) is a technology using a high pressure gasifier to turn coal and other carbon based fuels into pressurized gas—synthesis gas (syngas). It can then remove impurities from the syngas prior to the electricity generation cycle. Some of these pollutants, such as sulfur, can be turned into re-usable byproducts through the Claus process. This results in lower emissions of sulfur dioxide, particulates, mercury, and in some cases carbon dioxide. With additional process equipment, a water-gas shift reaction can increase gasification efficiency and reduce carbon monoxide emissions by converting it to carbon dioxide. The resulting carbon dioxide from the shift reaction can be separated, compressed, and stored through sequestration. Excess heat from the primary combustion and syngas fired generation is then passed to a steam cycle, similar to a combined cycle gas turbine. This process results in improved thermodynamic efficiency, compared to conventional pulverized coal combustion.
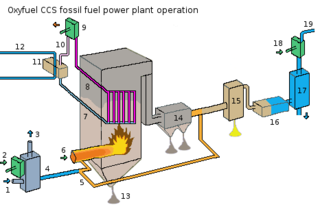
Oxy-fuel combustion is the process of burning a fuel using pure oxygen, or a mixture of oxygen and recirculated flue gas, instead of air. Since the nitrogen component of air is not heated, fuel consumption is reduced, and higher flame temperatures are possible. Historically, the primary use of oxy-fuel combustion has been in welding and cutting of metals, especially steel, since oxy-fuel allows for higher flame temperatures than can be achieved with an air-fuel flame. It has also received a lot of attention in recent decades as a potential carbon capture and storage technology.
Second-generation biofuels, also known as advanced biofuels, are fuels that can be manufactured from various types of non-food biomass. Biomass in this context means plant materials and animal waste used especially as a source of fuel.

Algae fuel, algal biofuel, or algal oil is an alternative to liquid fossil fuels that uses algae as its source of energy-rich oils. Also, algae fuels are an alternative to commonly known biofuel sources, such as corn and sugarcane. When made from seaweed (macroalgae) it can be known as seaweed fuel or seaweed oil.
Hydromethanation, [hahy-droh- meth-uh-ney-shuhn] is the process by which methane is produced through the combination of steam, carbonaceous solids and a catalyst in a fluidized bed reactor. The process, developed over the past 60 years by multiple research groups, enables the highly efficient conversion of coal, petroleum coke and biomass into clean, pipeline quality methane.

Bioenergy with carbon capture and storage (BECCS) is the process of extracting bioenergy from biomass and capturing and storing the carbon, thereby removing it from the atmosphere. BECCS can theoretically be a "negative emissions technology" (NET), although its deployment at the scale considered by many governments and industries can "also pose major economic, technological, and social feasibility challenges; threaten food security and human rights; and risk overstepping multiple planetary boundaries, with potentially irreversible consequences". The carbon in the biomass comes from the greenhouse gas carbon dioxide (CO2) which is extracted from the atmosphere by the biomass when it grows. Energy ("bioenergy") is extracted in useful forms (electricity, heat, biofuels, etc.) as the biomass is utilized through combustion, fermentation, pyrolysis or other conversion methods.
Carbon Sciences is a public corporation based in Santa Barbara. The company was founded in 2006 and incorporated as Zingerang, Inc. Originally, the company was involved in mobile communication, but has since switched to developing CO2 to fuel technology. Calcium carbonate, CaCO3, was briefly looked at as another end product of CO2 recycling. On April 2, 2007, the name was changed to Carbon Sciences Inc. Their process differs from other projects in that it does not utilize high pressure or high temperature. This would be a significant advantage when trying to scale the technology up to commercial production.
Carbon dioxide reforming is a method of producing synthesis gas from the reaction of carbon dioxide with hydrocarbons such as methane with the aid of noble metal catalysts. Synthesis gas is conventionally produced via the steam reforming reaction or coal gasification. In recent years, increased concerns on the contribution of greenhouse gases to global warming have increased interest in the replacement of steam as reactant with carbon dioxide.
Carbon-neutral fuel is fuel which produces no net-greenhouse gas emissions or carbon footprint. In practice, this usually means fuels that are made using carbon dioxide (CO2) as a feedstock. Proposed carbon-neutral fuels can broadly be grouped into synthetic fuels, which are made by chemically hydrogenating carbon dioxide, and biofuels, which are produced using natural CO2-consuming processes like photosynthesis.

Direct air capture (DAC) is the use of chemical or physical processes to extract carbon dioxide directly from the ambient air. If the extracted CO2 is then sequestered in safe long-term storage, the overall process will achieve carbon dioxide removal and be a "negative emissions technology" (NET).
Carbon tech is a group of existing and emerging technologies that are rapidly transforming oil and gas to low emissions energy. Combined, these technologies take a circular carbon economy approach for managing and reducing carbon footprints, while optimizing biological and industry processes. It builds on the principles of the circular economy for managing carbon emissions: to reduce the amount of carbon emissions entering the atmosphere, to reuse carbon emissions as a feedstock in different industries, to recycle carbon through the natural carbon cycle with bioenergy, and to remove carbon and store it. Carbon tech provides a third option for climate and environmental policy as an alternate to the binary business as usual and radical change.















