Related Research Articles
In biology, caveolae, which are a special type of lipid raft, are small invaginations of the plasma membrane, and the most abundant surface feature of, many vertebrate cell types in many vertebrate cell types, especially in endothelial cells, adipocytes and embryonic notochord cells. They were originally discovered by E. Yamada in 1955.

Dysferlin also known as dystrophy-associated fer-1-like protein is a protein that in humans is encoded by the DYSF gene.
Perlecan (PLC) also known as basement membrane-specific heparan sulfate proteoglycan core protein (HSPG) or heparan sulfate proteoglycan 2 (HSPG2), is a protein that in humans is encoded by the HSPG2 gene.

Caveolin-3 is a protein that in humans is encoded by the CAV3 gene. Alternative splicing has been identified for this locus, with inclusion or exclusion of a differentially spliced intron. In addition, transcripts utilize multiple polyA sites and contain two potential translation initiation sites.

Dystroglycan is a protein that in humans is encoded by the DAG1 gene.

Utrophin is a protein that in humans is encoded by the UTRN gene.

Low-density lipoprotein receptor-related protein 8 (LRP8), also known as apolipoprotein E receptor 2 (ApoER2), is a protein that in humans is encoded by the LRP8 gene. ApoER2 is a cell surface receptor that is part of the low-density lipoprotein receptor family. These receptors function in signal transduction and endocytosis of specific ligands. Through interactions with one of its ligands, reelin, ApoER2 plays an important role in embryonic neuronal migration and postnatal long-term potentiation. Another LDL family receptor, VLDLR, also interacts with reelin, and together these two receptors influence brain development and function. Decreased expression of ApoER2 is associated with certain neurological diseases.
Originally identified as Kirsten ras associated gene (krag), Sarcospan (SSPN) is a 25-kDa transmembrane protein located in the dystrophin-associated protein complex of skeletal muscle cells, where it is most abundant. It contains four transmembrane spanning helices with both N- and C-terminal domains located intracellularly. Loss of SSPN expression occurs in patients with Duchenne muscular dystrophy. Dystrophin is required for proper localization of SSPN. SSPN is also an essential regulator of Akt signaling pathways. Without SSPN, Akt signaling pathways will be hindered and muscle regeneration will not occur.
Dystrobrevin is a protein that binds to dystrophin in the costamere of skeletal muscle cells. In humans, there are at least two isoforms of dystrobrevin, dystrobrevin alpha and dystrobrevin beta.

Caveolin-1 is a protein that in humans is encoded by the CAV1 gene.
RAS p21 protein activator 1 or RasGAP, also known as RASA1, is a 120-kDa cytosolic human protein that provides two principal activities:

KH domain-containing, RNA-binding, signal transduction-associated protein 1 is a protein that in humans is encoded by the KHDRBS1 gene.

Laminin subunit alpha-2 is a protein that in humans is encoded by the LAMA2 gene.

Alpha-7 integrin is a protein that in humans is encoded by the ITGA7 gene. Alpha-7 integrin is critical for modulating cell-matrix interactions. Alpha-7 integrin is highly expressed in cardiac muscle, skeletal muscle and smooth muscle cells, and localizes to Z-disc and costamere structures. Mutations in ITGA7 have been associated with congenital myopathies and noncompaction cardiomyopathy, and altered expression levels of alpha-7 integrin have been identified in various forms of muscular dystrophy.

Flotillin-1 is a protein that in humans is encoded by the FLOT1 gene.

Flotillin-2 is a protein that in humans is encoded by the FLOT2 gene. Flotillin 2 (flot-2) is a highly conserved protein isolated from caveolae/lipid raft domains that tether growth factor receptors linked to signal transduction pathways. Flot-2 binds to PAR-1, a known upstream mediator of major signal transduction pathways implicated in cell growth and metastasis, and may influence tumour progression.

Caveolin-2 is a protein that in humans is encoded by the CAV2 gene.

Guanine nucleotide-binding protein subunit alpha-11 is a protein that in humans is encoded by the GNA11 gene. Together with GNAQ, it functions as a Gq alpha subunit.
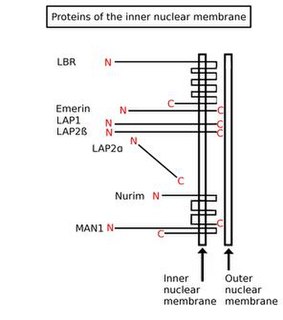
Inner nuclear membrane proteins are membrane proteins that are embedded in or associated with the inner membrane of the nuclear envelope. There are about 60 INM proteins, most of which are poorly characterized with respect to structure and function. Among the few well-characterized INM proteins are lamin B receptor (LBR), lamina-associated polypeptide 1 (LAP1), lamina-associated polypeptide-2 (LAP2), emerin and MAN1.
Patched (Ptc) is a conserved 12-pass transmembrane protein receptor that plays an obligate negative regulatory role in the Hedgehog signaling pathway in insects and vertebrates. Patched is an essential gene in embryogenesis for proper segmentation in the fly embryo, mutations in which may be embryonic lethal. Patched functions as the receptor for the Hedgehog protein and controls its spatial distribution, in part via endocytosis of bound Hedgehog protein, which is then targeted for lysosomal degradation.
References
- ↑ Tang Z, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS, Nishimoto I, Lodish HF, Lisanti MP (January 1996). "Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle". J. Biol. Chem. 271 (4): 2255–61. doi: 10.1074/jbc.271.4.2255 . PMID 8567687.
- ↑ Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP (January 1996). "Identification, sequence, and expression of caveolin-2 defines a caveolin gene family". Proc. Natl. Acad. Sci. U.S.A. 93 (1): 131–5. doi: 10.1073/pnas.93.1.131 . PMC 40192 . PMID 8552590.
- ↑ Williams TM, Lisanti MP (2004). "The caveolin proteins". Genome Biol. 5 (3): 214. doi:10.1186/gb-2004-5-3-214. PMC 395759 . PMID 15003112.
- ↑ Shatz M, Liscovitch M (March 2008). "Caveolin-1: a tumor-promoting role in human cancer". Int. J. Radiat. Biol. 84 (3): 177–89. doi:10.1080/09553000701745293. PMID 18300018. S2CID 23034625.
- ↑ Williams TM, Lisanti MP (2004). "The Caveolin genes: from cell biology to medicine". Ann. Med. 36 (8): 584–95. doi:10.1080/07853890410018899. PMID 15768830. S2CID 35611697.
- ↑ Vatta M, Ackerman MJ, Ye B, Makielski JC, Ughanze EE, Taylor EW, Tester DJ, Balijepalli RC, Foell JD, Li Z, Kamp TJ, Towbin JA (November 2006). "Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome". Circulation. 114 (20): 2104–12. doi: 10.1161/CIRCULATIONAHA.106.635268 . PMID 17060380.
- ↑ Galbiati F, Razani B, Lisanti MP (October 2001). "Caveolae and caveolin-3 in muscular dystrophy". Trends Mol Med. 7 (10): 435–41. doi:10.1016/S1471-4914(01)02105-0. PMID 11597517.