Related Research Articles

A vaccine is a biological preparation that provides active acquired immunity to a particular infectious disease. A vaccine typically contains an agent that resembles a disease-causing microorganism and is often made from weakened or killed forms of the microbe, its toxins, or one of its surface proteins. The agent stimulates the body's immune system to recognize the agent as a threat, destroy it, and to further recognize and destroy any of the microorganisms associated with that agent that it may encounter in the future. Vaccines can be prophylactic, or therapeutic. Some vaccines offer full sterilizing immunity, in which infection is prevented completely.

Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Unlike most antibiotics, antiviral drugs do not destroy their target pathogen; instead they inhibit its development.

Influenza vaccines, also known as flu jabs or flu shots, are vaccines that protect against infection by influenza viruses. New versions of the vaccines are developed twice a year, as the influenza virus rapidly changes. While their effectiveness varies from year to year, most provide modest to high protection against influenza. The United States Centers for Disease Control and Prevention (CDC) estimates that vaccination against influenza reduces sickness, medical visits, hospitalizations, and deaths. Immunized workers who do catch the flu return to work half a day sooner on average. Vaccine effectiveness in those over 65 years old remains uncertain due to a lack of high-quality research. Vaccinating children may protect those around them.
Live attenuated influenza vaccine (LAIV) is a type of influenza vaccine in the form of a nasal spray that is recommended for the prevention of influenza.

Amantadine, sold under the brand name Gocovri among others, is a medication used to treat dyskinesia associated with parkinsonism and influenza caused by type A influenzavirus, though its use for the latter is no longer recommended due to widespread drug resistance. It acts as a nicotinic antagonist, dopamine agonist, and noncompetitive NMDA antagonist. The antiviral mechanism of action is antagonism of the influenzavirus A M2 proton channel, which prevents endosomal escape.
Virus-like particles (VLPs) are molecules that closely resemble viruses, but are non-infectious because they contain no viral genetic material. They can be naturally occurring or synthesized through the individual expression of viral structural proteins, which can then self assemble into the virus-like structure. Combinations of structural capsid proteins from different viruses can be used to create recombinant VLPs. Both in-vivo assembly and in-vitro assembly have been successfully shown to form virus-like particles. VLPs derived from the Hepatitis B virus (HBV) and composed of the small HBV derived surface antigen (HBsAg) were described in 1968 from patient sera. VLPs have been produced from components of a wide variety of virus families including Parvoviridae, Retroviridae, Flaviviridae, Paramyxoviridae and bacteriophages. VLPs can be produced in multiple cell culture systems including bacteria, mammalian cell lines, insect cell lines, yeast and plant cells.
Virotherapy is a treatment using biotechnology to convert viruses into therapeutic agents by reprogramming viruses to treat diseases. There are three main branches of virotherapy: anti-cancer oncolytic viruses, viral vectors for gene therapy and viral immunotherapy. These branches use three different types of treatment methods: gene overexpression, gene knockout, and suicide gene delivery. Gene overexpression adds genetic sequences that compensate for low to zero levels of needed gene expression. Gene knockout uses RNA methods to silence or reduce expression of disease-causing genes. Suicide gene delivery introduces genetic sequences that induce an apoptotic response in cells, usually to kill cancerous growths. In a slightly different context, virotherapy can also refer more broadly to the use of viruses to treat certain medical conditions by killing pathogens.
Modified Vaccinia Ankara (MVA) is an attenuated (weakened) strain of the vaccinia virus. It is being used as a vaccine against smallpox and monkeypox, having fewer side effects than the traditional smallpox vaccine. By inserting antigen genes into its genome, modified vaccinia virus Ankara is also used as an experimental viral vector for vaccines against non-poxvirus diseases.

Peramivir is an antiviral drug developed by BioCryst Pharmaceuticals for the treatment of influenza. Peramivir is a neuraminidase inhibitor, acting as a transition-state analogue inhibitor of influenza neuraminidase and thereby preventing new viruses from emerging from infected cells. It is approved for intravenous administration.

Umifenovir, sold under the brand name Arbidol, is an antiviral medication for the treatment of influenza infection used in Russia and China. The drug is manufactured by Pharmstandard. Russian and China studies have shown it to be effective and it is approved in both countries while, it is not approved by the US FDA for the treatment or prevention of influenza because it was never applied for FDA approval since the drug company is in Russia not the US.
Rintatolimod, sold under the tradename Ampligen, is a medication intended for treatment of chronic fatigue syndrome (CFS). There is some evidence it may improve some CFS symptoms.

Hepatitis B vaccine is a vaccine that prevents hepatitis B. The first dose is recommended within 24 hours of birth with either two or three more doses given after that. This includes those with poor immune function such as from HIV/AIDS and those born premature. It is also recommended that health-care workers be vaccinated. In healthy people routine immunization results in more than 95% of people being protected.

Hepatitis A vaccine is a vaccine that prevents hepatitis A. It is effective in around 95% of cases and lasts for at least twenty years and possibly a person's entire life. If given, two doses are recommended beginning after the age of one. It is given by injection into a muscle. The first hepatitis A vaccine was approved in Europe in 1991, and the United States in 1995. It is on the World Health Organization's List of Essential Medicines.
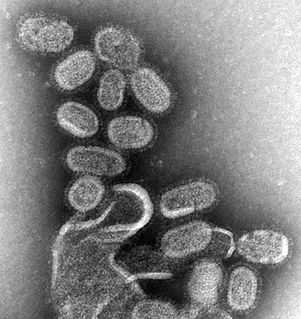
Influenza, commonly known as "the flu", is an infectious disease caused by influenza viruses. Symptoms range from mild to severe and often include fever, runny nose, sore throat, muscle pain, headache, coughing, and fatigue. These symptoms begin from one to four days after exposure to the virus and last for about 2–8 days. Diarrhea and vomiting can occur, particularly in children. Influenza may progress to pneumonia, which can be caused by the virus or by a subsequent bacterial infection. Other complications of infection include acute respiratory distress syndrome, meningitis, encephalitis, and worsening of pre-existing health problems such as asthma and cardiovascular disease.
Protein Sciences Corporation is a biotech company based in Meriden, Connecticut. The company develops and produces vaccines and biopharmaceuticals for use against influenza and other diseases. In 2017, the company was acquired by Sanofi for $750 million.

The 2009 swine flu pandemic vaccines were influenza vaccines developed to protect against the pandemic H1N1/09 virus. These vaccines either contained inactivated (killed) influenza virus, or weakened live virus that could not cause influenza. The killed virus was injected, while the live virus was given as a nasal spray. Both these types of vaccine were produced by growing the virus in chicken eggs. Around three billion doses were produced, with delivery in November 2009.

A H5N1 vaccine is an influenza vaccine intended to provide immunization to influenza A virus subtype H5N1.

A viral vector vaccine is a vaccine that uses a viral vector to deliver genetic material coding for a desired antigen into the recipient's host cells. As of April 2021, six viral vector vaccines have been authorized for use in humans in at least one country: four COVID-19 vaccines and two Ebola vaccines.
Type A influenza vaccine is for the prevention of infection of influenza A virus and also the influenza-related complications. Different monovalent type A influenza vaccines have been developed for different subtypes of influenza A virus including H1N1 and H5N1. Both intramuscular injection or intranasal spray are available on market. Unlike the seasonal influenza vaccines which are used annually, they are usually used during the outbreak of certain strand of subtypes of influenza A. Common adverse effects includes injection site reaction and local tenderness. Incidences of headache and myalgia were also reported with H1N1 whereas cases of fever has also been demonstrated with H5N1 vaccines. It is stated that immunosuppressant therapies would reduce the therapeutic effects of vaccines and that people with egg allergy should go for the egg-free preparations.
References
- 1 2 3 4 5 Audsley JM, Tannock GA (1 August 2008). "Cell-based influenza vaccines: progress to date". Drugs. 68 (11): 1483–91. doi:10.2165/00003495-200868110-00002. PMID 18627206. S2CID 46960558.
- ↑ Wong SS, Webby RJ (July 2013). "Traditional and new influenza vaccines". Clinical Microbiology Reviews. American Society for Microbiology. 26 (3): 476–92. doi:10.1128/cmr.00097-12. PMC 3719499 . PMID 23824369.
- ↑ "Vaccines Protect You". Vaccines.gov. Retrieved 18 December 2018.
- ↑ Nabel GJ (February 2013). "Designing tomorrow's vaccines". The New England Journal of Medicine. 368 (6): 551–60. doi:10.1056/nejmra1204186. PMC 3612922 . PMID 23388006.
- ↑ Vlecken DH, Pelgrim RP, Ruminski S, Bakker WA, van der Pol LA (October 2013). "Comparison of initial feasibility of host cell lines for viral vaccine production". Journal of Virological Methods. 193 (1): 28–41. doi:10.1016/j.jviromet.2013.04.020. PMID 23684847.
- 1 2 "How Influenza (Flu) Vaccines Are Made". Centers for Disease Control and Prevention (CDC). 24 September 2018. Retrieved 18 December 2018.
- 1 2 Perdue ML, Arnold F, Li S, Donabedian A, Cioce V, Warf T, Huebner R (August 2011). "The future of cell culture-based influenza vaccine production". Expert Review of Vaccines. 10 (8): 1183–94. doi:10.1586/erv.11.82. PMID 21854311. S2CID 28477882.
- 1 2 Zahoor MA, Khurshid M, Qureshi R, Naz A, Shahid M (July 2016). "Cell culture-based viral vaccines: current status and future prospects". Future Virology. 11 (7): 549–62. doi:10.2217/fvl-2016-0006.
- ↑ Audsley JM, Tannock GA (2008). "Cell-based influenza vaccines: progress to date". Drugs. 68 (11): 1483–91. doi:10.2165/00003495-200868110-00002. PMID 18627206. S2CID 46960558.
- ↑ "FDA clears first cell-based flu vaccine". Center for Infectious Disease Research and Policy. 21 November 2012. Retrieved 24 September 2013.[ verification needed ]
- ↑ "Vaccine Production in Cells". Flu.gov. 2006-07-17. Retrieved 2013-09-24.^[verification needed] [ verification needed ]
- 1 2 "Cell-Based Flu Vaccines". Centers for Disease Control and Prevention (CDC). 4 October 2018. Retrieved 19 December 2018.
- 1 2 Milián E, Kamen AA (2015). "Current and emerging cell culture manufacturing technologies for influenza vaccines". BioMed Research International. 2015: 504831. doi: 10.1155/2015/504831 . PMC 4359798 . PMID 25815321.
- ↑ "FDA approves new seasonal influenza vaccine made using novel technology" (Press release). U.S. Food and Drug Administration (FDA). 16 January 2013. Archived from the original on 18 May 2013.
- ↑ "FDA approves first flu vaccine grown in insect cells". CIDRAP. 14 October 2019. Archived from the original on 14 October 2019. Retrieved 14 October 2019.
- ↑ "Flublok". U.S. Food and Drug Administration (FDA). 26 February 2018. STN 125285. Archived from the original on 14 October 2019. Retrieved 14 October 2019.
- ↑ "Flublok Quadrivalent". U.S. Food and Drug Administration (FDA). 2 August 2019. STN 125285. Archived from the original on 14 October 2019. Retrieved 14 October 2019.
- ↑ "20 November 2012 Approval Letter- Flucelvax". U.S. Food and Drug Administration (FDA). 20 November 2012. Archived from the original on 23 July 2017. Retrieved 19 August 2017.
- ↑ "Summary Basis of Regulatory Action". Food and Drug Administration (FDA). 23 May 2016. Retrieved 27 June 2019.
Flucelvax was approved for active immunization against influenza for use in adults 18 years of age and older on 20 November 2012.
- ↑ "FDA approves first seasonal influenza vaccine manufactured using cell culture technology" (Press release). U.S. Food and Drug Administration (FDA). 20 November 2012. Archived from the original on 2 January 2013.
- ↑ Center for Biologics Evaluation and Research. "Approved Products – 20 November 2012 Approval Letter- Flucelvax". U.S. Food and Drug Administration (FDA). Archived from the original on 3 December 2012.
- 1 2 "Flucelvax Quadrivalent". U.S. Food and Drug Administration (FDA). 19 September 2019. STN BL 125408. Archived from the original on 17 October 2019. Retrieved 16 October 2019.
- ↑ "Flucelvax Product Information" (PDF). Food and Drug Administration (FDA). February 2013. Retrieved 10 November 2013.
- 1 2 Doroshenko A, Halperin SA (June 2009). "Trivalent MDCK cell culture-derived influenza vaccine Optaflu (Novartis Vaccines)". Expert Review of Vaccines. Informa UK Limited. 8 (6): 679–88. doi:10.1586/erv.09.31. PMID 19485748. S2CID 207223652.
- ↑ "Summary Basis of Regulatory Action" (PDF). Food and Drug Administration (FDA). 20 November 2012. Archived from the original (PDF) on 11 March 2016. Retrieved 10 September 2015.
The main differences in manufacturing between Flucelvax and Optaflu are limited to minor differences in release specifications and the methods used to calculate HA concentration.
- 1 2 3 "Is Egg-based Vaccine Manufacturing on its Way Out?". The Cell Culture Dish. 4 October 2011. Retrieved 9 October 2017.
- ↑ Toovey S (November 2007). "Preventing rabies with the Verorab vaccine: 1985-2005 Twenty years of clinical experience". Travel Medicine and Infectious Disease. 5 (6): 327–48. doi:10.1016/j.tmaid.2007.07.004. PMID 17983973.
- ↑ "Ixiaro". European Medicines Agency. 15 March 2019. Retrieved 27 June 2019.
The virus in Ixiaro is grown in mammal cells ('Vero cells') under laboratory conditions.