
Praseodymium(IV) oxide is an inorganic compound with chemical formula PrO2.
Terbium(IV) oxide is an inorganic compound with a chemical formula TbO2. It can be produced by oxidizing terbium(III) oxide by oxygen gas at 1000 atm and 300 °C.

Cerium(IV) fluoride is an inorganic compound with a chemical formula CeF4. It is a strong oxidant that appears as a white crystalline material. Cerium(IV) fluoride has an anhydrous form and a monohydrate form.
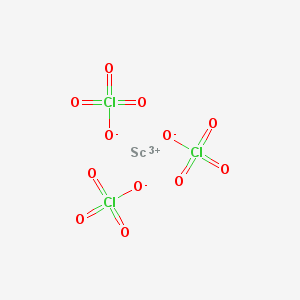
Scandium perchlorate is an inorganic compound with the chemical formula Sc(ClO4)3.

Neodymium(III) hydroxide is an insoluble inorganic compound with the chemical formula Nd(OH)3.

Samarium(III) hydroxide is an inorganic compound with chemical formula Sm(OH)3.
Thulium(II) chloride is an inorganic compound with the chemical formula TmCl2.

Europium(III) hydroxide is an inorganic compound with a chemical formula Eu(OH)3.

Erbium(III) hydroxide is an inorganic compound with chemical formula Er(OH)3.

Dysprosium(III) hydroxide is an inorganic compound with the chemical formula Dy(OH)3.

Thulium(III) hydroxide is an inorganic compound with the chemical formula Tm(OH)3.

Terbium(III) hydroxide is an inorganic compound with chemical formula Tb(OH)3.

Terbium(IV) fluoride is an inorganic compound with a chemical formula TbF4. It is a white solid that is a strong oxidizer. It is also a strong fluorinating agent, emitting relatively pure atomic fluorine when heated, rather than the mixture of fluoride vapors emitted from cobalt(III) fluoride or cerium(IV) fluoride. It can be produced by the reaction between very pure terbium(III) fluoride and xenon difluoride, chlorine trifluoride or fluorine gas:
Praseodymium(III) carbonate is an inorganic compound, with a chemical formula of Pr2(CO3)3. The anhydrous form is olive green, and many of its hydrates such as heptahydrate and octahydrate are known. They are all insoluble in water.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.

Terbium compounds are compounds formed by the lanthanide metal terbium (Tb). Terbium generally exhibits the +3 oxidation state in these compounds, such as in TbCl3, Tb(NO3)3 and Tb(CH3COO)3. Compounds with terbium in the +4 oxidation state are also known, such as TbO2 and BaTbF6. Terbium can also form compounds in the 0, +1 and +2 oxidation states.

Lutetium(III) iodide or lutetium iodide is an inorganic compound consisting of iodine and lutetium, with the chemical formula of LuI3.

Cerium acetate is an inorganic compound with the chemical formula of Ce(CH3COO)3. It is a white powder that is soluble in water. Its 1.5 hydrate loses water at 133°C to obtain an amorphous anhydrous form, and the amorphous phase changes to crystal at 212°C, and phase changes again at 286°C.

Holmium(III) hydroxide is an inorganic compound with the chemical formula Ho(OH)3.
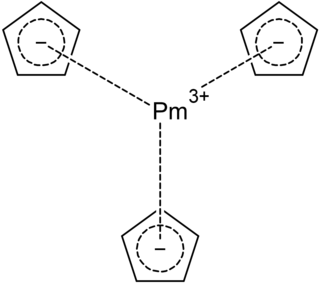
Promethocene is a metal-organic compound of promethium with the chemical formula Pm(C5H5)3. It is radioactive and stable in dry air. Promethocene is different from cyclopentadiene complexes of general transition metals and is considered to be ionically bonded. Theoretical calculations show that its Pm natural population analysis (NPA) electronic configuration is 6s0.115d1.194f2.21.

















