
In chemistry, an acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their application in solving related problems; these are called the acid–base theories, for example, Brønsted–Lowry acid–base theory.

Magnesium carbonate, MgCO3, is an inorganic salt that is a colourless or white solid. Several hydrated and basic forms of magnesium carbonate also exist as minerals.
The Brønsted–Lowry theory (also called proton theory of acids and bases) is an acid–base reaction theory which was first developed by Johannes Nicolaus Brønsted and Thomas Martin Lowry independently in 1923. The basic concept of this theory is that when an acid and a base react with each other, the acid forms its conjugate base, and the base forms its conjugate acid by exchange of a proton (the hydrogen cation, or H+). This theory generalises the Arrhenius theory.

Cerium(IV) sulfate, also called ceric sulfate, is an inorganic compound. It exists as the anhydrous salt Ce(SO4)2 as well as a few hydrated forms: Ce(SO4)2(H2O)x, with x equal to 4, 8, or 12. These salts are yellow to yellow/orange solids that are moderately soluble in water and dilute acids. Its neutral solutions slowly decompose, depositing the light yellow oxide CeO2. Solutions of ceric sulfate have a strong yellow color. The tetrahydrate loses water when heated to 180-200 °C.
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may need to be brought to this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, precipitation and other visible changes.
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. The reverse of disproportionation, such as when a compound in an intermediate oxidation state is formed from precursors of lower and higher oxidation states, is called comproportionation, also known as synproportionation.

Lead(II,IV) oxide, also called red lead or minium, is the inorganic compound with the formula Pb3O4. A bright red or orange solid, it is used as pigment, in the manufacture of batteries, and rustproof primer paints. It is an example of a mixed valence compound, being composed of both Pb(II) and Pb(IV) in the ratio of two to one.
Potassium hypomanganate is the inorganic compound with the formula K3MnO4. Also known as potassium manganate(V), this bright blue solid is a rare example of a salt with the hypomanganate or manganate(V) anion, where the manganese atom is in the +5 oxidation state. It is an intermediate in the production of potassium permanganate and the industrially most important Mn(V) compound.

Nickel(II) carbonate describes one or a mixture of inorganic compounds containing nickel and carbonate. From the industrial perspective, an important nickel carbonate is basic nickel carbonate with the formula Ni4CO3(OH)6(H2O)4. Simpler carbonates, ones more likely encountered in the laboratory, are NiCO3 and its hexahydrate. All are paramagnetic green solids containing Ni2+ cations. The basic carbonate is an intermediate in the hydrometallurgical purification of nickel from its ores and is used in electroplating of nickel.

Lithium peroxide is the inorganic compound with the formula Li2O2. Lithium peroxide is a white solid, and unlike most other alkali metal peroxides, it is nonhygroscopic. Because of its high oxygen:mass and oxygen:volume ratios, the solid has been used to remove CO2 from and release O2 to the atmosphere in spacecraft.

Cobalt(II) carbonate is the inorganic compound with the formula CoCO3. This reddish paramagnetic solid is an intermediate in the hydrometallurgical purification of cobalt from its ores. It is an inorganic pigment, and a precursor to catalysts. Cobalt(II) carbonate also occurs as the rare red/pink mineral spherocobaltite.
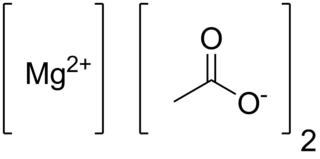
Anhydrous magnesium acetate has the chemical formula Mg(C2H3O2)2 and in its hydrated form, magnesium acetate tetrahydrate, it has the chemical formula Mg(CH3COO)2 • 4H2O. In this compound magnesium has an oxidation state of 2+. Magnesium acetate is the magnesium salt of acetic acid. It is deliquescent and upon heating, it decomposes to form magnesium oxide. Magnesium acetate is commonly used as a source of magnesium in biological reactions.

Magnesium bicarbonate or magnesium hydrogencarbonate, Mg(HCO3)2, is the bicarbonate salt of magnesium. It can be formed through the reaction of dilute solutions of carbonic acid (such as seltzer water) and magnesium hydroxide (milk of magnesia).

A carbonate fluoride, fluoride carbonate, fluorocarbonate or fluocarbonate is a double salt containing both carbonate and fluoride. The salts are usually insoluble in water, and can have more than one kind of metal cation to make more complex compounds. Rare-earth fluorocarbonates are particularly important as ore minerals for the light rare-earth elements lanthanum, cerium and neodymium. Bastnäsite is the most important source of these elements. Other artificial compounds are under investigation as non-linear optical materials and for transparency in the ultraviolet, with effects over a dozen times greater than Potassium dideuterium phosphate.

Neodymium(III) hydroxide is an insoluble inorganic compound with the chemical formula Nd(OH)3.
Neodymium(III) carbonate is an inorganic compound, a salt, where neodymium is in the +3 oxidation state and the carbonate ion has charge -2. It has a chemical formula of Nd2(CO3)3. The anhydrous form is purple-red, while the octahydrate is a pink solid. Both of these salts are insoluble in water.

Europium(III) acetate is an inorganic salt of europium and acetic acid with the chemical formula of Eu(CH3COO)3. In this compound, europium exhibits the +3 oxidation state. It can exist in the anhydrous form, sesquihydrate and tetrahydrate. Its hydrate molecule is a dimer.

Holmium acetate is the acetate salt of holmium, with a chemical formula of Ho(CH3COO)3.

Cerium acetate is an inorganic compound with the chemical formula of Ce(CH3COO)3. It is a white powder that is soluble in water. Its 1.5 hydrate loses water at 133°C to obtain an amorphous anhydrous form, and the amorphous phase changes to crystal at 212°C, and phase changes again at 286°C.














