Lanthanum is a chemical element with the symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between lanthanum and lutetium in the periodic table, of which lanthanum is the first and the prototype. Lanthanum is traditionally counted among the rare earth elements. Like most other rare earth elements, the usual oxidation state is +3, although some compounds are known with oxidation state +2. Lanthanum has no biological role in humans but is essential to some bacteria. It is not particularly toxic to humans but does show some antimicrobial activity.

Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na+ and hydroxide anions OH−.
Solubility equilibrium is a type of dynamic equilibrium that exists when a chemical compound in the solid state is in chemical equilibrium with a solution of that compound. The solid may dissolve unchanged, with dissociation, or with chemical reaction with another constituent of the solution, such as acid or alkali. Each solubility equilibrium is characterized by a temperature-dependent solubility product which functions like an equilibrium constant. Solubility equilibria are important in pharmaceutical, environmental and many other scenarios.
In chemistry, an amphoteric compound is a molecule or ion that can react both as an acid and as a base. What exactly this can mean depends on which definitions of acids and bases are being used.

Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca(OH)2. It is a colorless crystal or white powder and is produced when quicklime (calcium oxide) is mixed with water. It has many names including hydrated lime, caustic lime, builders' lime, slaked lime, cal, and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E526. Limewater, also called milk of lime, is the common name for a saturated solution of calcium hydroxide.

Lanthanum(III) oxide, also known as lanthana, chemical formula La2O3, is an inorganic compound containing the rare earth element lanthanum and oxygen. It is used in some ferroelectric materials, as a component of optical materials, and is a feedstock for certain catalysts, among other uses.

Silver oxide is the chemical compound with the formula Ag2O. It is a fine black or dark brown powder that is used to prepare other silver compounds.

Iron(III) oxide-hydroxide or ferric oxyhydroxide is the chemical compound of iron, oxygen, and hydrogen with formula FeO(OH).
A metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [M(H2O)n]z+. The solvation number, n, determined by a variety of experimental methods is 4 for Li+ and Be2+ and 6 for most elements in periods 3 and 4 of the periodic table. Lanthanide and actinide aqua ions have higher solvation numbers (often 8 to 9), with the highest known being 11 for Ac3+. The strength of the bonds between the metal ion and water molecules in the primary solvation shell increases with the electrical charge, z, on the metal ion and decreases as its ionic radius, r, increases. Aqua ions are subject to hydrolysis. The logarithm of the first hydrolysis constant is proportional to z2/r for most aqua ions.

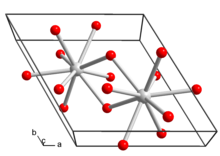
Lanthanum(III) nitrate is a water soluble salt of lanthanum with the chemical formula La(NO
3)
3. The compound decomposes at 499°C to lanthanum oxide, nitric oxide and oxygen.

Beryllium oxalate is an inorganic compound, a salt of beryllium metal and oxalic acid with the chemical formula C
2BeO
4. It forms colorless crystals, dissolves in water, and also forms crystalline hydrates. The compound is used to prepare ultra-pure beryllium oxide by thermal decomposition.
Samarium(III) oxalate is an inorganic compound, a salt of samarium and oxalic acid with the formula Sm2(C2O4)3. The compound does not dissolve in water, forms a crystalline hydrate with yellow crystals.
Neptunium(IV) nitrate is an inorganic compound, a salt of neptunium and nitric acid with the chemical formula Np(NO3)4. The compound forms gray crystals, dissolves in water, and forms crystal hydrates.
Dysprosium(III) nitrate is an inorganic compound, a salt of dysprosium and nitric acid with the chemical formula Dy(NO3)3. The compound forms yellowish crystals, dissolves in water, forms a crystalline hydrate.

Holmium (III) nitrate is an inorganic compound, a salt of holmium and nitric acid with the chemical formula Ho(NO3)3. The compound forms yellowish crystals, dissolves in water, also forms crystalline hydrates.

Ytterbium(III) nitrate is an inorganic compound, a salt of ytterbium and nitric acid with the chemical formula Yb(NO3)3. The compound forms colorless crystals, dissolves in water, and also forms crystalline hydrates.
Lutetium(III) nitrate is an inorganic compound, a salt of lutetium and nitric acid with the chemical formula Lu(NO3)3. The compound forms colorless crystals, dissolves in water, and also forms crystalline hydrates. The compound is poisonous.
Erbium(III) nitrate is an inorganic compound, a salt of erbium and nitric acid with the chemical formula Er(NO3)3. The compound forms pink crystals, readily soluble in water, also forms crystalline hydrates.

Praseodymium(III) acetate is an inorganic salt composed of a Praseodymium atom trication and three acetate groups as anions. This compound commonly forms the dihydrate, Pr(O2C2H3)3·2H2O.
Lanthanum oxalate is an inorganic compound, a salt of lanthanum metal and oxalic acid with the chemical formula La
2(C
2O
4)
3.