
Adhesive, also known as glue, cement, mucilage, or paste, is any non-metallic substance applied to one or both surfaces of two separate items that binds them together and resists their separation.

Hot-melt adhesive (HMA), also known as hot glue, is a form of thermoplastic adhesive that is commonly sold as solid cylindrical sticks of various diameters designed to be applied using a hot glue gun. The gun uses a continuous-duty heating element to melt the plastic glue, which the user pushes through the gun either with a mechanical trigger mechanism on the gun, or with direct finger pressure. The glue squeezed out of the heated nozzle is initially hot enough to burn and even blister skin. The glue is sticky when hot, and solidifies in a few seconds to one minute. Hot-melt adhesives can also be applied by dipping or spraying, and are popular with hobbyists and crafters both for affixing and as an inexpensive alternative to resin casting.
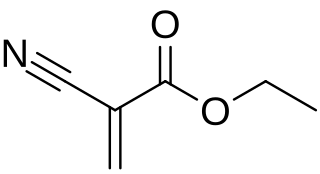
Ethyl cyanoacrylate (ECA), a cyanoacrylate ester, is an ethyl ester of 2-cyano-acrylic acid. It is a colorless liquid with low viscosity and a faint sweet smell in pure form. It is the main component of cyanoacrylate glues and can be encountered under many trade names. It is soluble in acetone, methyl ethyl ketone, nitromethane, and methylene chloride. ECA polymerizes rapidly in presence of moisture.

Harry Wesley Coover Jr. was the inventor of Eastman 910, commonly known as Super Glue.
An acrylate polymer is any of a group of polymers prepared from acrylate monomers. These plastics are noted for their transparency, resistance to breakage, and elasticity.
Wood glue is an adhesive used to tightly bond pieces of wood together. Many substances have been used as glues. Traditionally animal proteins like casein from milk or collagen from animal hides and bones were boiled down to make early glues. They worked by solidifying as they dried. Later, glues were made from plant starches like flour or potato starch. When combined with water and heated, the starch gelatinizes and forms a sticky paste as it dries. Plant-based glues were common for books and paper products, though they can break down more easily over time compared to animal-based glues. Examples of modern wood glues include polyvinyl acetate (PVA) and epoxy resins. Some resins used in producing composite wood products may contain formaldehyde. As of 2021, “the wood panel industry uses almost 95% of synthetic petroleum-derived thermosetting adhesives, mainly based on urea, phenol, and melamine, among others”.

Elmer's Products, Inc. or simply Elmer's, is an American-based company that has a line of adhesive, craft, home repair, and office supply products. It is best known as the manufacturer of Elmer's Glue-All, a popular PVA-based synthetic glue, in addition to other brands including Krazy Glue, ProBond and CraftBond adhesives, and X-Acto cutting tools.
Loctite is an American brand of adhesives, sealants, surface treatments, and other industrial chemicals that include acrylic, anaerobic, cyanoacrylate, epoxy, hot melt, silicone, urethane, and UV/light curing technologies. Loctite products are sold globally and are used in a variety of industrial and hobbyist applications.
Liquid bandage is a topical skin treatment for minor wounds which binds to the skin to form a protective polymeric layer that keeps dirt and germs out and moisture in.

A surgical suture, also known as a stitch or stitches, is a medical device used to hold body tissues together and approximate wound edges after an injury or surgery. Application generally involves using a needle with an attached length of thread. There are numerous types of suture which differ by needle shape and size as well as thread material and characteristics. Selection of surgical suture should be determined by the characteristics and location of the wound or the specific body tissues being approximated.

Wound closure strips are porous surgical tape strips which can be used to close small wounds. They are applied across the laceration in a manner which pulls the skin on either side of the wound together. Wound closure strips may be used instead of sutures (stitches) in some injuries, because they lessen scarring and are easier to care for.
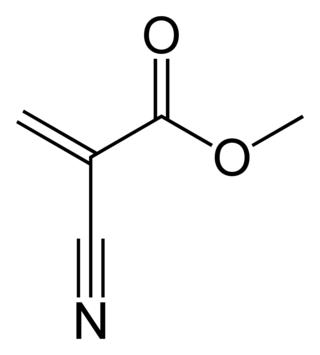
Methyl cyanoacrylate is an organic compound that contains several functional groups: a methyl ester, a nitrile, and an alkene. It is a colorless liquid with low viscosity. Its chief use is as the main component of cyanoacrylate glues. It can be encountered under many trade names. Methyl cyanoacrylate is less commonly encountered than ethyl cyanoacrylate.

n-Butyl cyanoacrylate, a cyanoacrylate ester, is a butyl ester of 2-cyano-2-propenoic acid. It is a colorless liquid with a sharp, irritating odor. It is insoluble in water. Its chief use is as the main component of medical cyanoacrylate glues. It can be encountered under various trade names, e.g. Cutseal, MediBond, MediCryl, PeriAcryl, GluStitch, Xoin, Gesika, VetGlu, Vetbond, LiquiVet, Indermil, LiquiBand, Histoacryl, IFABond, CutisSeal and others. The generic international nonproprietary name (INN) for NBCA is enbucrilate.

Octyl cyanoacrylate (OCA), a cyanoacrylate ester, is an octyl ester of 2-cyano-2-propenoic acid. It is a clear colorless liquid with a sharp, irritating odor. Its chief use is as the main component of medical cyanoacrylate glues.

Artificial nails, also known as fake nails, false nails, acrylic nails, nail extensions or nail enhancements, are extensions placed over fingernails as fashion accessories. Many artificial nail designs attempt to mimic the appearance of real fingernails as closely as possible, while others may deliberately stray in favor of an artistic look.
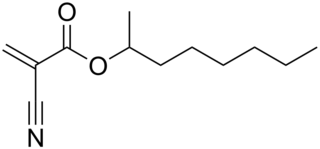
2-Octyl cyanoacrylate is a cyanoacrylate ester typically used as a wound closure adhesive. It is closely related to octyl cyanoacrylate. The use of 2-octyl cyanoacrylate was approved in 1998; offered as an alternative to stitches, sutures, and or adhesive strips.
Hydrophobic light-activated adhesive (HLAA) is a type of glue that sets in seconds, but only after exposure to ultraviolet light. One biocompatible, biodegradable HLAA is under consideration for use in human tissue repair as a replacement for sutures, staples and other approaches.

Chemence is a speciality chemical and medical device manufacturer which products include cyanoacrylate adhesives, anaerobic adhesives, impregnation sealants, adhesive activators, epoxy resins, UV adhesives, photopolymer resins, custom printer ink cartridges, Gas pipe sealants, and soak off nail polishes. Chemence is a supplier of photopolymer and commercial printers to the flexographic industry in the US and Europe, as well as the primary sealant supplier to British Gas, and a primary supplier of private-label adhesives to companies including Tesco, 3M, Bostik, and Bondo. The company's catalogue of patents includes processes, packaging devices, and chemical combinations.
Adhesive bonding is a joining technique used in the manufacture and repair of a wide range of products. Along with welding and soldering, adhesive bonding is one of the basic joining processes. In this technique, components are bonded together using adhesives. The broad range of types of adhesives available allows numerous materials to be bonded together in products as diverse as vehicles, mobile phones, personal care products, buildings, computers and medical devices.



















