The actinide or actinoid series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.

Nuclear reprocessing is the chemical separation of fission products and actinides from spent nuclear fuel. Originally, reprocessing was used solely to extract plutonium for producing nuclear weapons. With commercialization of nuclear power, the reprocessed plutonium was recycled back into MOX nuclear fuel for thermal reactors. The reprocessed uranium, also known as the spent fuel material, can in principle also be re-used as fuel, but that is only economical when uranium supply is low and prices are high. Nuclear reprocessing may extend beyond fuel and include the reprocessing of other nuclear reactor material, such as Zircaloy cladding.

Nuclear chemistry is the sub-field of chemistry dealing with radioactivity, nuclear processes, and transformations in the nuclei of atoms, such as nuclear transmutation and nuclear properties.
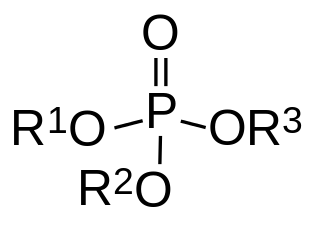
In organic chemistry, organophosphates are a class of organophosphorus compounds with the general structure O=P(OR)3, a central phosphate molecule with alkyl or aromatic substituents. They can be considered as esters of phosphoric acid. Organophosphates are best known for their use as pesticides.
Hydrometallurgy is a technique within the field of extractive metallurgy, the obtaining of metals from their ores. Hydrometallurgy involve the use of aqueous solutions for the recovery of metals from ores, concentrates, and recycled or residual materials. Processing techniques that complement hydrometallurgy are pyrometallurgy, vapour metallurgy, and molten salt electrometallurgy. Hydrometallurgy is typically divided into three general areas:
Tributyl phosphate, known commonly as TBP, is an organophosphorus compound with the chemical formula (CH3CH2CH2CH2O)3PO. This colourless, odorless liquid finds some applications as an extractant and a plasticizer. It is an ester of phosphoric acid with n-butanol.
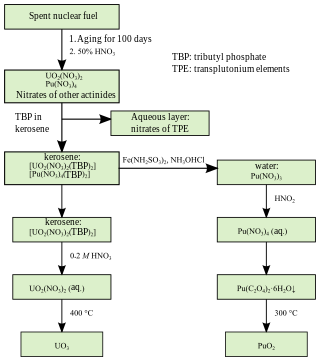
PUREX is a chemical method used to purify fuel for nuclear reactors or nuclear weapons. PUREX is the de facto standard aqueous nuclear reprocessing method for the recovery of uranium and plutonium from used nuclear fuel. It is based on liquid–liquid extraction ion-exchange.

Liquid–liquid extraction, also known as solvent extraction and partitioning, is a method to separate compounds or metal complexes, based on their relative solubilities in two different immiscible liquids, usually water (polar) and an organic solvent (non-polar). There is a net transfer of one or more species from one liquid into another liquid phase, generally from aqueous to organic. The transfer is driven by chemical potential, i.e. once the transfer is complete, the overall system of chemical components that make up the solutes and the solvents are in a more stable configuration. The solvent that is enriched in solute(s) is called extract. The feed solution that is depleted in solute(s) is called the raffinate. Liquid-liquid extraction is a basic technique in chemical laboratories, where it is performed using a variety of apparatus, from separatory funnels to countercurrent distribution equipment called as mixer settlers. This type of process is commonly performed after a chemical reaction as part of the work-up, often including an acidic work-up.

Uranyl nitrate is a water-soluble yellow uranium salt with the formula UO2(NO3)2 · n H2O. The hexa-, tri-, and dihydrates are known. The compound is mainly of interest because it is an intermediate in the preparation of nuclear fuels. In the nuclear industry, it is commonly referred to as yellow salt.
The uranyl ion is an oxycation of uranium in the oxidation state +6, with the chemical formula UO2+
2. It has a linear structure with short U–O bonds, indicative of the presence of multiple bonds between uranium and oxygen. Four or more ligands may be bound to the uranyl ion in an equatorial plane around the uranium atom. The uranyl ion forms many complexes, particularly with ligands that have oxygen donor atoms. Complexes of the uranyl ion are important in the extraction of uranium from its ores and in nuclear fuel reprocessing.

Uranyl chloride refers to inorganic compounds with the formula UO2Cl2(H2O)n where n = 0, 1, or 3. These are yellow-colored salts.

Uranium tetrachloride is an inorganic compound, a salt of uranium and chlorine, with the formula UCl4. It is a hygroscopic olive-green solid. It was used in the electromagnetic isotope separation (EMIS) process of uranium enrichment. It is one of the main starting materials for organouranium chemistry.

2-Ethylhexanol is an organic compound with the chemical formula CH3CH2CH2CH2CH(CH2CH3)CH2OH. It is a branched, eight-carbon chiral alcohol. It is a colorless liquid that is poorly soluble in water but soluble in most organic solvents. It is produced on a large scale (>2,000,000,000 kg/y) for use in numerous applications such as solvents, flavors, and fragrances and especially as a precursor for production of other chemicals such as emollients and plasticizers. It is encountered in plants, fruits, and wines. The odor has been reported as "heavy, earthy, and slightly floral" for the R enantiomer and "a light, sweet floral fragrance" for the S enantiomer.
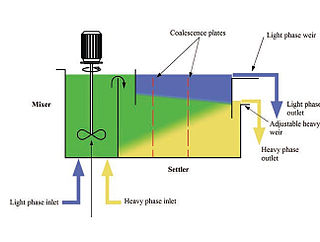
Mixer settlers are a class of mineral process equipment used in the solvent extraction process. A mixer settler consists of a first stage that mixes the phases together followed by a quiescent settling stage that allows the phases to separate by gravity.

The bismuth-phosphate process was used to extract plutonium from irradiated uranium taken from nuclear reactors. It was developed during World War II by Stanley G. Thompson, a chemist working for the Manhattan Project at the University of California, Berkeley. This process was used to produce plutonium at the Hanford Site. Plutonium was used in the atomic bomb that was used in the atomic bombing of Nagasaki in August 1945. The process was superseded in the 1950s by the REDOX and PUREX processes.
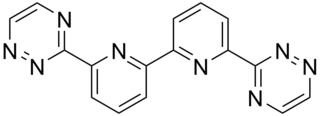
The bis-triazinyl bipyridines (BTBPs) are a class of chemical compounds which are tetradentate ligands similar in shape to quaterpyridine. The BTBPs are made by the reaction of hydrazine and a 1,2-diketone with 6,6'-dicyano-2,2'-bipyridine. The dicyanobipy can be made by reacting 2,2'-bipy with hydrogen peroxide in acetic acid, to form 2,2'-bipyridine-N,N-dioxide. The 2,2'-bipyridine-N,N-dioxide is then converted into the dicyano compound by treatment with potassium cyanide and benzoyl chloride in a mixture of water and THF.
The plutonyl ion is an oxycation of plutonium in the oxidation state +6, with the chemical formula PuO2+
2. It is isostructural with the uranyl ion, compared to which it has a slightly shorter M–O bond. It is easily reduced to plutonium(III). The plutonyl ion forms many complexes, particularly with ligands that have oxygen donor atoms. Plutonyl salts are important in nuclear fuel reprocessing.

Actinide chemistry is one of the main branches of nuclear chemistry that investigates the processes and molecular systems of the actinides. The actinides derive their name from the group 3 element actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide. All but one of the actinides are f-block elements, corresponding to the filling of the 5f electron shell; lawrencium, a d-block element, is also generally considered an actinide. In comparison with the lanthanides, also mostly f-block elements, the actinides show much more variable valence. The actinide series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium.
The advanced reprocessing of spent nuclear fuel is a potential key to achieve a sustainable nuclear fuel cycle and to tackle the heavy burden of nuclear waste management. In particular, the development of such advanced reprocessing systems may save natural resources, reduce waste inventory and enhance the public acceptance of nuclear energy. This strategy relies on the recycling of major actinides and the transmutation of minor actinides in appropriate reactors. In order to fulfill this objective, selective extracting agents need to be designed and developed by investigating their complexation mechanism.

Thulium(III) nitrate is an inorganic compound, a salt of thulium and nitric acid with the chemical formula Tm(NO3)3. The compound forms dark-green crystals, readily soluble in water, also forms crystalline hydrates.















