
The phrenic nerve is a mixed motor/sensory nerve that originates from the C3-C5 spinal nerves in the neck. The nerve is important for breathing because it provides exclusive motor control of the diaphragm, the primary muscle of respiration. In humans, the right and left phrenic nerves are primarily supplied by the C4 spinal nerve, but there is also a contribution from the C3 and C5 spinal nerves. From its origin in the neck, the nerve travels downward into the chest to pass between the heart and lungs towards the diaphragm.

The thoracic diaphragm, or simply the diaphragm, is a sheet of internal skeletal muscle in humans and other mammals that extends across the bottom of the thoracic cavity. The diaphragm is the most important muscle of respiration, and separates the thoracic cavity, containing the heart and lungs, from the abdominal cavity: as the diaphragm contracts, the volume of the thoracic cavity increases, creating a negative pressure there, which draws air into the lungs. Its high oxygen consumption is noted by the many mitochondria and capillaries present; more than in any other skeletal muscle.
The control of ventilation is the physiological mechanisms involved in the control of breathing, which is the movement of air into and out of the lungs. Ventilation facilitates respiration. Respiration refers to the utilization of oxygen and balancing of carbon dioxide by the body as a whole, or by individual cells in cellular respiration.

Central hypoventilation syndrome (CHS) is a sleep-related breathing disorder that causes ineffective breathing, apnea, or respiratory arrest during sleep. CHS can either be congenital (CCHS) or acquired (ACHS) later in life. The condition can be fatal if untreated. CCHS was once known as Ondine's curse.

Functional electrical stimulation (FES) is a technique that uses low-energy electrical pulses to artificially generate body movements in individuals who have been paralyzed due to injury to the central nervous system. More specifically, FES can be used to generate muscle contraction in otherwise paralyzed limbs to produce functions such as grasping, walking, bladder voiding and standing. This technology was originally used to develop neuroprostheses that were implemented to permanently substitute impaired functions in individuals with spinal cord injury (SCI), head injury, stroke and other neurological disorders. In other words, a person would use the device each time he or she wanted to generate a desired function. FES is sometimes also referred to as neuromuscular electrical stimulation (NMES).

Artificial ventilation is a means of assisting or stimulating respiration, a metabolic process referring to the overall exchange of gases in the body by pulmonary ventilation, external respiration, and internal respiration. It may take the form of manually providing air for a person who is not breathing or is not making sufficient respiratory effort, or it may be mechanical ventilation involving the use of a mechanical ventilator to move air in and out of the lungs when an individual is unable to breathe on their own, for example during surgery with general anesthesia or when an individual is in a coma or trauma.
A side stitch is an intense stabbing abdominal pain under the lower edge of the ribcage that occurs during exercise. It is also called a side ache, side cramp, muscle stitch, or simply stitch, and the medical term is exercise-related transient abdominal pain (ETAP). It sometimes extends to shoulder tip pain, and commonly occurs during running, swimming, and horseback riding. Approximately two-thirds of runners will experience at least one episode of a stitch each year. The precise cause is unclear, although it most likely involves irritation of the abdominal lining, and the condition is more likely after consuming a meal or a sugary beverage. If the pain is present only when exercising and is completely absent at rest, in an otherwise healthy person, it does not require investigation. Typical treatment strategies involve deep breathing and/or manual pressure on the affected area.

An implant is a medical device manufactured to replace a missing biological structure, support a damaged biological structure, or enhance an existing biological structure. For example, an implant may be a rod, used to strengthen weak bones. Medical implants are human-made devices, in contrast to a transplant, which is a transplanted biomedical tissue. The surface of implants that contact the body might be made of a biomedical material such as titanium, silicone, or apatite depending on what is the most functional. In 2018, for example, American Elements developed a nickel alloy powder for 3D printing robust, long-lasting, and biocompatible medical implants. In some cases implants contain electronics, e.g. artificial pacemaker and cochlear implants. Some implants are bioactive, such as subcutaneous drug delivery devices in the form of implantable pills or drug-eluting stents.
Neuroprosthetics is a discipline related to neuroscience and biomedical engineering concerned with developing neural prostheses. They are sometimes contrasted with a brain–computer interface, which connects the brain to a computer rather than a device meant to replace missing biological functionality.

The pericardiacophrenic artery is a long slender branch of the internal thoracic artery.

A spinal cord stimulator (SCS) or dorsal column stimulator (DCS) is a type of implantable neuromodulation device that is used to send electrical signals to select areas of the spinal cord for the treatment of certain pain conditions. SCS is a consideration for people who have a pain condition that has not responded to more conservative therapy. There are also spinal cord stimulators under research and development that could enable patients with spinal cord injury to walk again via epidural electrical stimulation (EES).
Sacral nerve stimulation, also termed sacral neuromodulation, is a type of medical electrical stimulation therapy.
Diaphragmatic paradox or paradoxical diaphragm phenomenon is an abnormal medical sign observed during respiration, in which the diaphragm moves opposite to the normal directions of its movements. The diaphragm normally moves downwards during inspiration and upwards during expiration. But in diaphragmatic paradox, it moves upwards during inspiration and downwards during expiration.
Neurally adjusted ventilatory assist (NAVA) is a mode of mechanical ventilation. NAVA delivers assistance in proportion to and in synchrony with the patient's respiratory efforts, as reflected by an electrical signal. This signal represents the electrical activity of the diaphragm, the body's principal breathing muscle.
Central sleep apnea (CSA) or central sleep apnea syndrome (CSAS) is a sleep-related disorder in which the effort to breathe is diminished or absent, typically for 10 to 30 seconds either intermittently or in cycles, and is usually associated with a reduction in blood oxygen saturation. CSA is usually due to an instability in the body's feedback mechanisms that control respiration. Central sleep apnea can also be an indicator of Arnold–Chiari malformation.
Neurostimulation is the purposeful modulation of the nervous system's activity using invasive or non-invasive means. Neurostimulation usually refers to the electromagnetic approaches to neuromodulation.
Neuromodulation is "the alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation or chemical agents, to specific neurological sites in the body". It is carried out to normalize – or modulate – nervous tissue function. Neuromodulation is an evolving therapy that can involve a range of electromagnetic stimuli such as a magnetic field (rTMS), an electric current, or a drug instilled directly in the subdural space. Emerging applications involve targeted introduction of genes or gene regulators and light (optogenetics), and by 2014, these had been at minimum demonstrated in mammalian models, or first-in-human data had been acquired. The most clinical experience has been with electrical stimulation.
Stentrode is a small stent-mounted electrode array permanently implanted into a blood vessel in the brain, without the need for open brain surgery. It is in clinical trials as a brain–computer interface (BCI) for people with paralyzed or missing limbs, who will use their neural signals or thoughts to control external devices, which currently include computer operating systems. The device may ultimately be used to control powered exoskeletons, robotic prosthesis, computers or other devices.
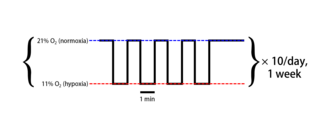
Intermittent hypoxia (also known as episodic hypoxia) is an intervention in which a person or animal undergoes alternating periods of normoxia and hypoxia. Normoxia is defined as exposure to oxygen levels normally found in Earth's atmosphere (~21% O2) and hypoxia as any oxygen levels lower than those of normoxia. Normally, exposure to hypoxia is negatively associated to physiological changes to the body, such as altitude sickness. However, when used in moderation, intermittent hypoxia may be used clinically as a means to alleviate various pathological conditions.
Neural dust is a hypothetical class of nanometer-sized devices operated as wirelessly powered nerve sensors; it is a type of brain–computer interface. The sensors may be used to study, monitor, or control the nerves and muscles and to remotely monitor neural activity. In practice, a medical treatment could introduce thousands of neural dust devices into human brains. The term is derived from "smart dust", as the sensors used as neural dust may also be defined by this concept.








