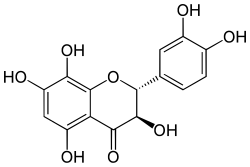
Flavonoids are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans.

Catechin is a flavan-3-ol, a type of secondary metabolite providing antioxidant roles in plants. It belongs to the subgroup of polyphenols called flavonoids.
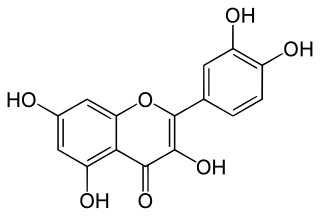
Quercetin is a plant flavonol from the flavonoid group of polyphenols. It is found in many fruits, vegetables, leaves, seeds, and grains; capers, red onions, and kale are common foods containing appreciable amounts of it. It has a bitter flavor and is used as an ingredient in dietary supplements, beverages, and foods.
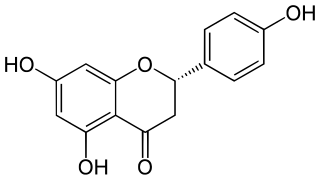
Naringenin is a flavorless, colorless flavanone, a type of flavonoid. It is the predominant flavanone in grapefruit, and is found in a variety of fruits and herbs.

Rutin, also called rutoside, quercetin-3-O-rutinoside and sophorin, is the glycoside combining the flavonol quercetin and the disaccharide rutinose. It is a flavonoid found in a wide variety of plants, including citrus.

Hesperidin is a flavanone glycoside found in citrus fruits. Its aglycone form is called hesperetin. Its name is derived from the word "hesperidium", for fruit produced by citrus trees.

Apigenin (4′,5,7-trihydroxyflavone), found in many plants, is a natural product belonging to the flavone class that is the aglycone of several naturally occurring glycosides. It is a yellow crystalline solid that has been used to dye wool.
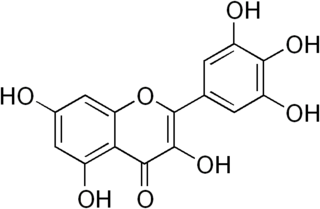
Myricetin is a member of the flavonoid class of polyphenolic compounds, with antioxidant properties. Common dietary sources include vegetables, fruits, nuts, berries, tea, and red wine. Myricetin is structurally similar to fisetin, luteolin, and quercetin and is reported to have many of the same functions as these other members of the flavonol class of flavonoids. Reported average intake of myricetin per day varies depending on diet, but has been shown in the Netherlands to average 23 mg/day.
In enzymology, a flavonoid 3'-monooxygenase (EC 1.14.14.82, was wrongly classified as EC 1.14.13.21 in the past) is an enzyme that catalyzes the chemical reaction
In enzymology, a taxifolin 8-monooxygenase (EC 1.14.13.19) is an enzyme that catalyzes the chemical reaction
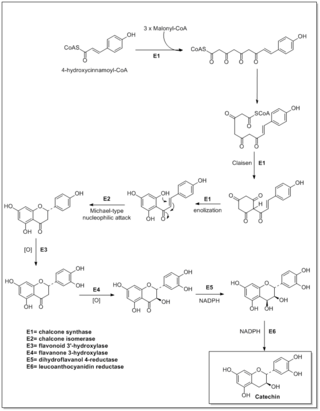
Flavonoids are synthesized by the phenylpropanoid metabolic pathway in which the amino acid phenylalanine is used to produce 4-coumaroyl-CoA. This can be combined with malonyl-CoA to yield the true backbone of flavonoids, a group of compounds called chalcones, which contain two phenyl rings. Conjugate ring-closure of chalcones results in the familiar form of flavonoids, the three-ringed structure of a flavone. The metabolic pathway continues through a series of enzymatic modifications to yield flavanones → dihydroflavonols → anthocyanins. Along this pathway, many products can be formed, including the flavonols, flavan-3-ols, proanthocyanidins (tannins) and a host of other various polyphenolics.

Anthocyanins, also called anthocyans, are water-soluble vacuolar pigments that, depending on their pH, may appear red, purple, blue, or black. In 1835, the German pharmacist Ludwig Clamor Marquart gave the name Anthokyan to a chemical compound that gives flowers a blue color for the first time in his treatise "Die Farben der Blüthen". Food plants rich in anthocyanins include the blueberry, raspberry, black rice, and black soybean, among many others that are red, blue, purple, or black. Some of the colors of autumn leaves are derived from anthocyanins.
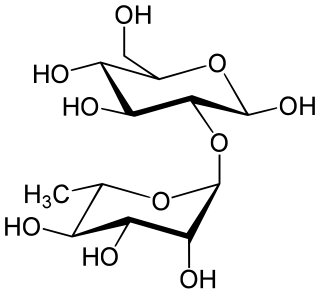
Neohesperidose is the disaccharide which is present in some flavonoids. It can be found in species of Typha.

The flavanonols are a class of flavonoids that use the 3-hydroxy-2,3-dihydro-2-phenylchromen-4-one backbone.

Taxifolin (5,7,3',4'-flavan-on-ol), also known as dihydroquercetin, belongs to the subclass flavanonols in the flavonoids, which in turn is a class of polyphenols.
The molecular formula C15H12O7 (molar mass: 304.25 g/mol, exact mass: 304.058303 u) may refer to:
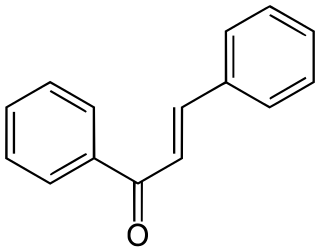
Chalconoids Greek: χαλκός khalkós, "copper", due to its color), also known as chalcones, are natural phenols related to chalcone. They form the central core for a variety of important biological compounds.

Smilax glabra, sarsaparilla, is a plant species in the genus Smilax. It is native to China, the Himalayas, and Indochina.
Flavonoid 3',5'-hydroxylase (EC 1.14.14.81 was wrongly classified as EC 1.14.13.88 in the past) is an enzyme with systematic name flavanone,NADPH:oxygen oxidoreductase. This enzyme catalyses the following chemical reaction
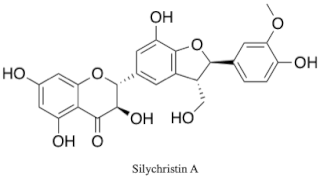
Silychristin is a natural product and one of the constituents of silymarin, the standardized, active extract of the fruit of milk thistle, Silybum marianum. It is the second most abundant constituent in silymarin, after silybin. Silychristin is a flavonolignan, along with many other silymarin constituents, meaning it is composed up of a flavonoid and a lignan. It is estimated that up to 65–80% of silymarin extract is made up of flavonolignans, like silychristin, which give silymarin its well known potent antioxidant and hepatoprotective properties. Silychristin can exist as two stereoisomers, silychristin A and silychristin B. The marianum variety of S. marianum includes silychristin A as a major flavonolignan constituent, while the lesser known and studied albiflorum variety includes unique flavonolignans, including silyhermin, (–)-silandrin, and (+)-silymonin.