Related Research Articles

The endometrium is the inner epithelial layer, along with its mucous membrane, of the mammalian uterus. It has a basal layer and a functional layer: the basal layer contains stem cells which regenerate the functional layer. The functional layer thickens and then is shed during menstruation in humans and some other mammals, including apes, Old World monkeys, some species of bat, the elephant shrew and the Cairo spiny mouse. In most other mammals, the endometrium is reabsorbed in the estrous cycle. During pregnancy, the glands and blood vessels in the endometrium further increase in size and number. Vascular spaces fuse and become interconnected, forming the placenta, which supplies oxygen and nutrition to the embryo and fetus. The speculated presence of an endometrial microbiota has been argued against.
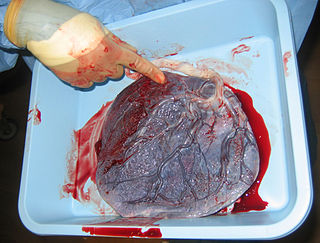
The placenta is a temporary embryonic and later fetal organ that begins developing from the blastocyst shortly after implantation. It plays critical roles in facilitating nutrient, gas and waste exchange between the physically separate maternal and fetal circulations, and is an important endocrine organ, producing hormones that regulate both maternal and fetal physiology during pregnancy. The placenta connects to the fetus via the umbilical cord, and on the opposite aspect to the maternal uterus in a species-dependent manner. In humans, a thin layer of maternal decidual (endometrial) tissue comes away with the placenta when it is expelled from the uterus following birth. Placentas are a defining characteristic of placental mammals, but are also found in marsupials and some non-mammals with varying levels of development.
Luteinizing hormone is a hormone produced by gonadotropic cells in the anterior pituitary gland. The production of LH is regulated by gonadotropin-releasing hormone (GnRH) from the hypothalamus. In females, an acute rise of LH known as an LH surge, triggers ovulation and development of the corpus luteum. In males, where LH had also been called interstitial cell–stimulating hormone (ICSH), it stimulates Leydig cell production of testosterone. It acts synergistically with follicle-stimulating hormone (FSH).

Human chorionic gonadotropin (hCG) is a hormone for the maternal recognition of pregnancy produced by trophoblast cells that are surrounding a growing embryo, which eventually forms the placenta after implantation. The presence of hCG is detected in some pregnancy tests. Some cancerous tumors produce this hormone; therefore, elevated levels measured when the patient is not pregnant may lead to a cancer diagnosis and, if high enough, paraneoplastic syndromes, however, it is not known whether this production is a contributing cause, or an effect of carcinogenesis. The pituitary analog of hCG, known as luteinizing hormone (LH), is produced in the pituitary gland of males and females of all ages.

The blastocyst is a structure formed in the early embryonic development of mammals. It possesses an inner cell mass (ICM) also known as the embryoblast which subsequently forms the embryo, and an outer layer of trophoblast cells called the trophectoderm. This layer surrounds the inner cell mass and a fluid-filled cavity known as the blastocoel. In the late blastocyst the trophectoderm is known as the trophoblast. The trophoblast gives rise to the chorion and amnion, the two fetal membranes that surround the embryo. The placenta derives from the embryonic chorion and the underlying uterine tissue of the mother.
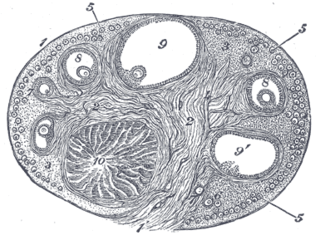
The corpus luteum is a temporary endocrine structure in female ovaries involved in the production of relatively high levels of progesterone, and moderate levels of estradiol, and inhibin A. It is the remains of the ovarian follicle that has released a mature ovum during a previous ovulation.
Gonadotropins are glycoprotein hormones secreted by gonadotropic cells of the anterior pituitary of vertebrates. This family includes the mammalian hormones follicle-stimulating hormone (FSH) and luteinizing hormone (LH), the placental/chorionic gonadotropins, human chorionic gonadotropin (hCG) and equine chorionic gonadotropin (eCG), as well as at least two forms of fish gonadotropins. These hormones are central to the complex endocrine system that regulates normal growth, sexual development, and reproductive function. LH and FSH are secreted by the anterior pituitary gland, while hCG and eCG are secreted by the placenta in pregnant humans and mares, respectively. The gonadotropins act on the gonads, controlling gamete and sex hormone production.

The trophoblast is the outer layer of cells of the blastocyst. Trophoblasts are present four days after fertilization in humans. They provide nutrients to the embryo and develop into a large part of the placenta. They form during the first stage of pregnancy and are the first cells to differentiate from the fertilized egg to become extraembryonic structures that do not directly contribute to the embryo. After blastulation, the trophoblast is contiguous with the ectoderm of the embryo and is referred to as the trophectoderm. After the first differentiation, the cells in the human embryo lose their totipotency because they can no longer form a trophoblast. They become pluripotent stem cells.

Embryonic diapause (delayed implantation in mammals) is a reproductive strategy used by a number of animal species across different biological classes. In more than 130 types of mammals where this takes place, the process occurs at the blastocyst stage of embryonic development, and is characterized by a dramatic reduction or complete cessation of mitotic activity, arresting most often in the G0 or G1 phase of division.

Syncytiotrophoblast is the epithelial covering of the highly vascular embryonic placental villi, which invades the wall of the uterus to establish nutrient circulation between the embryo and the mother. It is a multinucleate, terminally differentiated syncytium, extending to 13 cm.

Human placental lactogen (hPL), also called human chorionic somatomammotropin (hCS) or human chorionic somatotropin, is a polypeptide placental hormone, the human form of placental lactogen. Its structure and function are similar to those of human growth hormone. It modifies the metabolic state of the mother during pregnancy to facilitate energy supply to the fetus. hPL has anti-insulin properties. hPL is a hormone secreted by the syncytiotrophoblast during pregnancy. Like human growth hormone, hPL is encoded by genes on chromosome 17q22-24. It was identified in 1963.

"Cytotrophoblast" is the name given to both the inner layer of the trophoblast or the cells that live there. It is interior to the syncytiotrophoblast and external to the wall of the blastocyst in a developing embryo.

Implantation, also known as nidation is the stage in the embryonic development of mammals in which the blastocyst hatches, attaches, adheres, and invades into the wall of the female's uterus. Implantation is the first stage of gestation, and, when successful, the female is considered to be pregnant. An implanted embryo is detected by the presence of increased levels of human chorionic gonadotropin (hCG) in a pregnancy test. The implanted embryo will receive oxygen and nutrients in order to grow.

Decidualization is a process that results in significant changes to cells of the endometrium in preparation for, and during, pregnancy. This includes morphological and functional changes to endometrial stromal cells (ESCs), the presence of decidual white blood cells (leukocytes), and vascular changes to maternal arteries. The sum of these changes results in the endometrium changing into a structure called the decidua. In humans, the decidua is shed during childbirth.

Human embryonic development, or human embryogenesis, is the development and formation of the human embryo. It is characterised by the processes of cell division and cellular differentiation of the embryo that occurs during the early stages of development. In biological terms, the development of the human body entails growth from a one-celled zygote to an adult human being. Fertilization occurs when the sperm cell successfully enters and fuses with an egg cell (ovum). The genetic material of the sperm and egg then combine to form the single cell zygote and the germinal stage of development commences. Embryonic development in the human, covers the first eight weeks of development; at the beginning of the ninth week the embryo is termed a fetus. The eight weeks has 23 stages.

Lactation describes the secretion of milk from the mammary glands and the period of time that a mother lactates to feed her young. The process naturally occurs with all sexually mature female mammals, although it may predate mammals. The process of feeding milk in all female creatures is called nursing, and in humans it is also called breastfeeding. Newborn infants often produce some milk from their own breast tissue, known colloquially as witch's milk.
Breast development, also known as mammogenesis, is a complex biological process in primates that takes place throughout a female's life.
Chorionic gonadotropin, beta polypeptide 5 is a protein that in humans is encoded by the CGB5 gene.

Maternal recognition of pregnancy is a crucial aspect of carrying a pregnancy to full term. Without maternal recognition to maintain pregnancy, the initial messengers which stop luteolysis and promote foetal implantation, growth and uterine development finish with nothing to replace them and the pregnancy is lost.
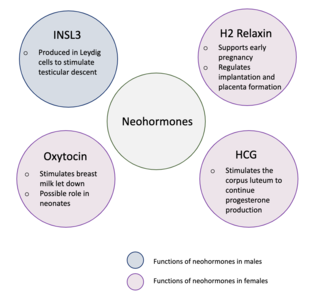
Neohormones are a group of recently evolved hormones primarily associated to the success of mammalian development. These hormones are specific to mammals and are not found in other vertebrates—this is because neohormones are evolved to enhance specific mammalian functions. In males, neohormones play important roles in regulating testicular descent and preparing the sperm for internal fertilisation. In females, neohormones are essential for regulating early pregnancy, mammary gland development lactation, and viviparity. Neohormones superimpose their actions on the hypothalamic-pituitary-gonadal axis and are not associated with other core bodily functions.
References
- ↑ Zhuang, L., & Li, R. (1991). Study on reproductive endocrinology of human placenta (II): hormone secreting activity of cytotrophoblast cells. Sci China B., 34, 1092–1097.)
- ↑ Gerami-Naini, B. et al (2004). Trophoblast differentiation in embryoid bodies derived from human embryonic stem cells. Endocrinology, 145, 1517–1524.
- ↑ Pidoux, G. et al (2007). Biochemical characterization and modulation of LH/CG-receptor during human trophoblast differentiation. Journal of Cell Physiology, 212, 26–35.
- 1 2 Gallego, M. et al (2009). Opioid and progesterone signaling is obligatory for early human embryogenesis. Stem Cells Development, 18, 737–740.
- 1 2 3 Gallego, M. et al (2010). The pregnancy hormones human chorionic gonadotropin and progesterone induce human embryonic stem cell proliferation and differentiation into neuroectodermal rosettes. Stem Cell Research & Therapy, 1, 1-13
- ↑ Carr, B., MacDonald, P., Simpson, E. (1982). The role of lipoproteins in the regulation of progesterone secretion by the human corpus luteum. Fertil Steril, 38, 303-311
- ↑ Licht, P. et al (2007). Is human chorionic gonadotropin directly involved in the regulation of human implantation? Molecular and Cellular Endocrinology, 269, 85-92.
- ↑ Bahado-Singh, R., et al (2002). The role of hyperglycosylated hCG in trophoblast invasion and the prediction of subsequent pre-eclampsia. Prenatal Diagnosis, 22, 478-481.
- ↑ Ma, W. et al (2001). Adult Tissue Angiogenesis: Evidence for negative regulation by estrogen in the uterus. Molecular Endocrinology, 15, 1983-1992.
- ↑ Zygmunt M, Herr F, Keller-Schoenwetter S, Kunzi-Rapp K, Münstedt K, Rao CV, Lang U, Preissner KT (2002). Characterization of human chorionic gonadotropin as a novel angiogenic factor. J Clin Endocrinol Metab. 87, 5290-5296.
- ↑ Clemens, L., Siiteri, P., & Stites, D. (1979). Mechanism of immunosuppression of progesterone on maternal lymphocyte activation during pregnancy. The Journal of Immunology, 122, 1978-1985.
- ↑ Yen S, et al. Causal relationship between hormonal variables in the menstrual cycle. In Ferin M, Richart RM, Vande Wiele RL (eds). Biorhythms and Human Reproduction. New York, John Wiley and Sons, 1974, pp 219-238.
- ↑ Zeleznik, A., Fairchild Benyo, D. Control of follicular development, corpus luteum function and the recognition of pregnancy in higher primates. In Knobil E (ed). The Physiology of Reproduction. New York, Raven Press, 1994, pp 751-782.
- ↑ Sleiter, N., Pang, Y., Park, C., Horton, T., Dong, J., Thomas, P., & Levine, J. (2009). Progesterone Receptor A (PRA) and PRB-Independent Effects of Progesterone on Gonadotropin-Releasing Hormone Release. Endocrinology, 150, 3833-3844.
- ↑ Kalkhoff, R. (1982). Metabolic effects of progesterone. American Journal of Obstetrician Gynecology, 142, 735-738.
- 1 2 Atwood, C. et al (2000). Progesterone induces side-branching of the ductal epithelium in the mammary glands of peripubertal mice. Journal of Endocrinology, 167, 39-52.
- ↑ Fantl, V., Edwards, P., Steel, J., Vonderhaar, B., & Dickson, C. (1999). Impaired Mammary Gland Development in Cyl-12/2 Mice during Pregnancy and Lactation Is Epithelial Cell Autonomous. Developmental Biology, 212, 1–11.
- ↑ Rao, C. et al (1995). Peripheral and intracerebroventricular administration of human chorionic gonadotropin alters several hippocampus-associated behaviors in cycling female rats. Hormones and Behavior, 29, 42-58