
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement.

Structural biology, as defined by the Journal of Structural Biology, deals with structural analysis of living material at every level of organization. Early structural biologists throughout the 19th and early 20th centuries were primarily only able to study structures to the limit of the naked eye's visual acuity and through magnifying glasses and light microscopes.

The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. It is composed of three main components: microfilaments, intermediate filaments, and microtubules, and these are all capable of rapid growth or disassembly depending on the cell's requirements.

Tubulin in molecular biology can refer either to the tubulin protein superfamily of globular proteins, or one of the member proteins of that superfamily. α- and β-tubulins polymerize into microtubules, a major component of the eukaryotic cytoskeleton. Microtubules function in many essential cellular processes, including mitosis. Tubulin-binding drugs kill cancerous cells by inhibiting microtubule dynamics, which are required for DNA segregation and therefore cell division.
In cell biology, microtubule-associated proteins (MAPs) are proteins that interact with the microtubules of the cellular cytoskeleton. MAPs are integral to the stability of the cell and its internal structures and the transport of components within the cell.

Transmission electron cryomicroscopy (CryoTEM), commonly known as cryo-EM, is a form of cryogenic electron microscopy, more specifically a type of transmission electron microscopy (TEM) where the sample is studied at cryogenic temperatures. Cryo-EM, specifically 3-dimensional electron microscopy (3DEM), is gaining popularity in structural biology.

Cryogenic electron tomography (cryoET) is an imaging technique used to reconstruct high-resolution (~1–4 nm) three-dimensional volumes of samples, often biological macromolecules and cells. cryoET is a specialized application of transmission electron cryomicroscopy (CryoTEM) in which samples are imaged as they are tilted, resulting in a series of 2D images that can be combined to produce a 3D reconstruction, similar to a CT scan of the human body. In contrast to other electron tomography techniques, samples are imaged under cryogenic conditions. For cellular material, the structure is immobilized in non-crystalline, vitreous ice, allowing them to be imaged without dehydration or chemical fixation, which would otherwise disrupt or distort biological structures.
Katanin is a microtubule-severing AAA protein. It is named after the Japanese sword called a katana. Katanin is a heterodimeric protein first discovered in sea urchins. It contains a 60 kDa ATPase subunit, encoded by KATNA1, which functions to sever microtubules. This subunit requires ATP and the presence of microtubules for activation. The second 80 kDA subunit, encoded by KATNB1, regulates the activity of the ATPase and localizes the protein to centrosomes. Electron microscopy shows that katanin forms 14–16 nm rings in its active oligomerized state on the walls of microtubules.

In biology, a protein filament is a long chain of protein monomers, such as those found in hair, muscle, or in flagella. Protein filaments form together to make the cytoskeleton of the cell. They are often bundled together to provide support, strength, and rigidity to the cell. When the filaments are packed up together, they are able to form three different cellular parts. The three major classes of protein filaments that make up the cytoskeleton include: actin filaments, microtubules and intermediate filaments.
In cell biology, microtubule nucleation is the event that initiates de novo formation of microtubules (MTs). These filaments of the cytoskeleton typically form through polymerization of α- and β-tubulin dimers, the basic building blocks of the microtubule, which initially interact to nucleate a seed from which the filament elongates.
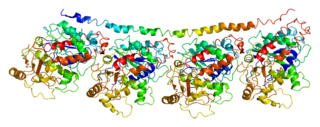
Tubulin alpha-1A chain is a protein that in humans is encoded by the TUBA1A gene.

Tubulin/FtsZ family, GTPase domain is an evolutionary conserved protein domain.
Susan Band Horwitz is an American biochemist and professor at the Albert Einstein College of Medicine where she holds the Falkenstein chair in Cancer Research as well as co-chair of the department of Molecular Pharmacology.

Cryogenic electron microscopy (cryo-EM) is a cryomicroscopy technique applied on samples cooled to cryogenic temperatures. For biological specimens, the structure is preserved by embedding in an environment of vitreous ice. An aqueous sample solution is applied to a grid-mesh and plunge-frozen in liquid ethane or a mixture of liquid ethane and propane. While development of the technique began in the 1970s, recent advances in detector technology and software algorithms have allowed for the determination of biomolecular structures at near-atomic resolution. This has attracted wide attention to the approach as an alternative to X-ray crystallography or NMR spectroscopy for macromolecular structure determination without the need for crystallization.

Lori Anne Passmore is a Canadian/British cryo electron microscopist and structural biologist who works at the Medical Research Council (MRC) Laboratory of Molecular Biology (LMB) at the University of Cambridge. She is known for her work on multiprotein complexes involved in gene expression and development of new supports for cryo-EM.

Tamir Gonen is an American structural biochemist and membrane biophysicist best known for his contributions to structural biology of membrane proteins, membrane biochemistry and electron cryo-microscopy (cryoEM) particularly in electron crystallography of 2D crystals and for the development of 3D electron crystallography from microscopic crystals known as MicroED. Gonen is an Investigator of the Howard Hughes Medical Institute, a professor at the University of California, Los Angeles, the founding director of the MicroED Imaging Center at UCLA and a Member of the Royal Society of New Zealand.

Kiyoshi Nagai was a Japanese structural biologist at the MRC Laboratory of Molecular Biology Cambridge, UK. He was known for his work on the mechanism of RNA splicing and structures of the spliceosome.
Mavis Agbandje-McKenna was a Nigerian-born British medical biophysicist, structural virologist, and a professor of structural biology, as well as the director of the Center for Structural Biology at the University of Florida in Gainesville, Florida. Agbandje-McKenna studied parvovirus structures using X-ray crystallography and cryogenic electron microscopy and did much of the initial work to elucidate the basic structure and function of adeno-associated viruses (AAVs). Her viral characterization and elucidation of antibody binding sites on AAV capsids has led to the development of viral capsid development and gene therapy approaches that evade immune detection and can be used to treat human diseases such as muscular dystrophies. Agbandje-McKenna was recognized with the 2020 American Society of Gene and Cell Therapy Outstanding Achievement Award for her contributions to the field. She died in 2021 from amyotrophic lateral sclerosis.
Antonina Roll-Mecak is a Romanian-born American molecular biophysicist. She is currently the Senior Investigator and Chief of the Unit of Cell Biology and Biophysics at the National Institutes of Health. She holds appointments at the National Institute of Neurological Disorders and Stroke and at the Biochemistry and Biophysics Center of the National Heart, Lung and Blood Institute. Roll-Mecak is known for her work on cytoskeletal regulation, mechanisms of microtubule severing enzymes and microtubule repair, and for her pioneering work in deciphering the complexities of the tubulin code. Her work is relevant to the treatment of cancer and nervous system disorders.
Gaia Pigino is the Associate Head of the Structural Biology Research Center and Research Group Leader of the Pigino Group at the Human Technopole in Milan, Italy.












