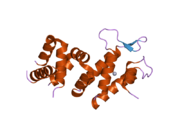
Breast cancer type 1 susceptibility protein is a protein that in humans is encoded by the BRCA1 gene. Orthologs are common in other vertebrate species, whereas invertebrate genomes may encode a more distantly related gene. BRCA1 is a human tumor suppressor gene and is responsible for repairing DNA.

Fanconi anemia (FA) is a rare, AR, genetic disease resulting in impaired response to DNA damage in the FA/BRCA pathway. Although it is a very rare disorder, study of this and other bone marrow failure syndromes has improved scientific understanding of the mechanisms of normal bone marrow function and development of cancer. Among those affected, the majority develop cancer, most often acute myelogenous leukemia (AML), MDS, and liver tumors. 90% develop aplastic anemia by age 40. About 60–75% have congenital defects, commonly short stature, abnormalities of the skin, arms, head, eyes, kidneys, and ears, and developmental disabilities. Around 75% have some form of endocrine problem, with varying degrees of severity. 60% of FA is FANC-A, 16q24.3, which has later onset bone marrow failure.

Glutathione S-transferase P is an enzyme that in humans is encoded by the GSTP1 gene.

Fanconi anemia group C protein is a protein that in humans is encoded by the FANCC gene. This protein delays the onset of apoptosis and promotes homologous recombination repair of damaged DNA. Mutations in this gene result in Fanconi anemia, a human rare disorder characterized by cancer susceptibility and cellular sensitivity to DNA crosslinks and other damages.

Fanconi anaemia, complementation group A, also known as FAA, FACA and FANCA, is a protein which in humans is encoded by the FANCA gene. It belongs to the Fanconi anaemia complementation group (FANC) family of genes of which 12 complementation groups are currently recognized and is hypothesised to operate as a post-replication repair or a cell cycle checkpoint. FANCA proteins are involved in inter-strand DNA cross-link repair and in the maintenance of normal chromosome stability that regulates the differentiation of haematopoietic stem cells into mature blood cells.

Fanconi anemia group D2 protein is a protein that in humans is encoded by the FANCD2 gene. The Fanconi anemia complementation group (FANC) currently includes FANCA, FANCB, FANCC, FANCD1, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCJ, FANCL, FANCM, FANCN and FANCO.

Fanconi anemia group G protein is a protein that in humans is encoded by the FANCG gene.
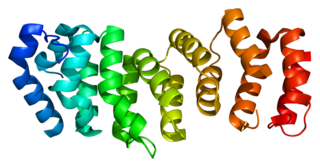
Fanconi anemia, complementation group E protein is a protein that in humans is encoded by the FANCE gene. The Fanconi anemia complementation group (FANC) currently includes FANCA, FANCB, FANCC, FANCD1, FANCD2, FANCE, FANCF, FANCG, and FANCL. Fanconi anemia is a genetically heterogeneous recessive disorder characterized by cytogenetic instability, hypersensitivity to DNA cross-linking agents, increased chromosomal breakage, and defective DNA repair. The members of the Fanconi anemia complementation group do not share sequence similarity; they are related by their assembly into a common nuclear protein complex. This gene encodes the protein for complementation groufcrp E.

E3 ubiquitin-protein ligase FANCL is an enzyme that in humans is encoded by the FANCL gene.

Fanconi anemia group B protein is a protein that in humans is encoded by the FANCB gene.

Fanconi anemia, complementation group I (FANCI) also known as KIAA1794, is a protein which in humans is encoded by the FANCI gene. Mutations in the FANCI gene are known to cause Fanconi anemia.

Partner and localizer of BRCA2, also known as PALB2 or FANCN, is a protein which in humans is encoded by the PALB2 gene.

Zinc finger and BTB domain-containing protein 32 is a protein that in humans is encoded by the 1960 bp ZBTB32 gene. The 52 kDa protein is a transcriptional repressor and the gene is expressed in T and B cells upon activation, but also significantly in testis cells. It is a member of the Poxviruses and Zinc-finger (POZ) and Krüppel (POK) family of proteins, and was identified in multiple screens involving either immune cell tumorigenesis or immune cell development.

Ubiquitin-specific protease 14 is an enzyme that in humans is encoded by the USP14 gene.

RecQ-mediated genome instability protein 1 is a protein that in humans is encoded by the RMI1 gene.

Fanconi anemia, complementation group M, also known as FANCM is a human gene. It is an emerging target in cancer therapy, in particular cancers with specific genetic deficiencies.
FANC proteins are a network of at least 15 proteins that are associated with a cell process known as the Fanconi anemia.
Alan D. D'Andrea is an American cancer researcher and the Fuller American Cancer Society Professor of Radiation Oncology at Harvard Medical School. D'Andrea's research at the Dana Farber Cancer Institute focuses on chromosome instability and cancer susceptibility. He is currently the director of the Center for DNA Damage and Repair and the director of the Susan F. Smith Center for Women's Cancer.
A hereditary cancer syndrome is a genetic disorder in which inherited genetic mutations in one or more genes predispose the affected individuals to the development of cancer and may also cause early onset of these cancers. Hereditary cancer syndromes often show not only a high lifetime risk of developing cancer, but also the development of multiple independent primary tumors.

Ketan Jayakrishna Patel is a British-Kenyan scientist who is Director of the MRC Weatherall Institute of Molecular Medicine and the MRC Molecular Haematology Unit at the University of Oxford. Until 2020 he was a tenured principal investigator at the Medical Research Council (MRC) Laboratory of Molecular Biology (LMB).