Related Research Articles
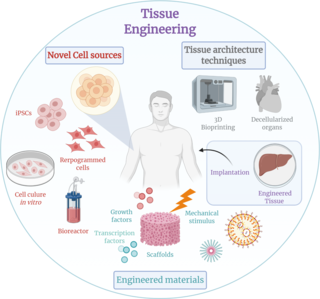
Tissue engineering is a biomedical engineering discipline that uses a combination of cells, engineering, materials methods, and suitable biochemical and physicochemical factors to restore, maintain, improve, or replace different types of biological tissues. Tissue engineering often involves the use of cells placed on tissue scaffolds in the formation of new viable tissue for a medical purpose but is not limited to applications involving cells and tissue scaffolds. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance it can be considered as a field of its own.

A hydrogel is a biphasic material, a mixture of porous, permeable solids and at least 10% by weight or volume of interstitial fluid composed completely or mainly by water . In hydrogels the porous permeable solid is a water insoluble three dimensional network of natural or synthetic polymers and a fluid, having absorbed a large amount of water or biological fluids. These properties underpin several applications, especially in the biomedical area. Many hydrogels are synthetic, but some are derived from nature. The term 'hydrogel' was coined in 1894.
Articular cartilage, most notably that which is found in the knee joint, is generally characterized by very low friction, high wear resistance, and poor regenerative qualities. It is responsible for much of the compressive resistance and load bearing qualities of the knee joint and, without it, walking is painful to impossible. Osteoarthritis is a common condition of cartilage failure that can lead to limited range of motion, bone damage and invariably, pain. Due to a combination of acute stress and chronic fatigue, osteoarthritis directly manifests itself in a wearing away of the articular surface and, in extreme cases, bone can be exposed in the joint. Some additional examples of cartilage failure mechanisms include cellular matrix linkage rupture, chondrocyte protein synthesis inhibition, and chondrocyte apoptosis. There are several different repair options available for cartilage damage or failure.
A biomaterial is a substance that has been engineered to interact with biological systems for a medical purpose, either a therapeutic or a diagnostic one. As a science, biomaterials is about fifty years old. The study of biomaterials is called biomaterials science or biomaterials engineering. It has experienced steady and strong growth over its history, with many companies investing large amounts of money into the development of new products. Biomaterials science encompasses elements of medicine, biology, chemistry, tissue engineering and materials science.
Cardiomyoplasty is a surgical procedure in which healthy muscle from another part of the body is wrapped around the heart to provide support for the failing heart. Most often the latissimus dorsi muscle is used for this purpose. A special pacemaker is implanted to make the skeletal muscle contract. If cardiomyoplasty is successful and increased cardiac output is achieved, it usually acts as a bridging therapy, giving time for damaged myocardium to be treated in other ways, such as remodeling by cellular therapies.
Neural tissue engineering is a specific sub-field of tissue engineering. Neural tissue engineering is primarily a search for strategies to eliminate inflammation and fibrosis upon implantation of foreign substances. Often foreign substances in the form of grafts and scaffolds are implanted to promote nerve regeneration and to repair damage caused to nerves of both the central nervous system (CNS) and peripheral nervous system (PNS) by an injury.
A nerve guidance conduit is an artificial means of guiding axonal regrowth to facilitate nerve regeneration and is one of several clinical treatments for nerve injuries. When direct suturing of the two stumps of a severed nerve cannot be accomplished without tension, the standard clinical treatment for peripheral nerve injuries is autologous nerve grafting. Due to the limited availability of donor tissue and functional recovery in autologous nerve grafting, neural tissue engineering research has focused on the development of bioartificial nerve guidance conduits as an alternative treatment, especially for large defects. Similar techniques are also being explored for nerve repair in the spinal cord but nerve regeneration in the central nervous system poses a greater challenge because its axons do not regenerate appreciably in their native environment.
Autologous chondrocyte implantation is a biomedical treatment that repairs damages in articular cartilage. ACI provides pain relief while at the same time slowing down the progression or considerably delaying partial or total joint replacement surgery. The goal of ACI is to allow people suffering from articular cartilage damage to return to their old lifestyle; regaining mobility, going back to work and even practicing sports again.

Fibrin glue is a surgical formulation used to create a fibrin clot for hemostasis, cartilage repair surgeries or wound healing. It contains separately packaged human fibrinogen and human thrombin.
Nano-scaffolding is a medical process used to regrow tissue and bone, including limbs and organs. The nano-scaffold is a three-dimensional structure composed of polymer fibers very small that are scaled from a Nanometer scale. Developed by the American military, the medical technology uses a microscopic apparatus made of fine polymer fibers called a scaffold. Damaged cells grip to the scaffold and begin to rebuild missing bone and tissue through tiny holes in the scaffold. As tissue grows, the scaffold is absorbed into the body and disappears completely.

Arginylglycylaspartic acid (RGD) is the most common peptide motif responsible for cell adhesion to the extracellular matrix (ECM), found in species ranging from Drosophila to humans. Cell adhesion proteins called integrins recognize and bind to this sequence, which is found within many matrix proteins, including fibronectin, fibrinogen, vitronectin, osteopontin, and several other adhesive extracellular matrix proteins. The discovery of RGD and elucidation of how RGD binds to integrins has led to the development of a number of drugs and diagnostics, while the peptide itself is used ubiquitously in bioengineering. Depending on the application and the integrin targeted, RGD can be chemically modified or replaced by a similar peptide which promotes cell adhesion.

Decellularization is the process used in biomedical engineering to isolate the extracellular matrix (ECM) of a tissue from its inhabiting cells, leaving an ECM scaffold of the original tissue, which can be used in artificial organ and tissue regeneration. Organ and tissue transplantation treat a variety of medical problems, ranging from end organ failure to cosmetic surgery. One of the greatest limitations to organ transplantation derives from organ rejection caused by antibodies of the transplant recipient reacting to donor antigens on cell surfaces within the donor organ. Because of unfavorable immune responses, transplant patients suffer a lifetime taking immunosuppressing medication. Stephen F. Badylak pioneered the process of decellularization at the McGowan Institute for Regenerative Medicine at the University of Pittsburgh. This process creates a natural biomaterial to act as a scaffold for cell growth, differentiation and tissue development. By recellularizing an ECM scaffold with a patient’s own cells, the adverse immune response is eliminated. Nowadays, commercially available ECM scaffolds are available for a wide variety of tissue engineering. Using peracetic acid to decellularize ECM scaffolds have been found to be false and only disinfects the tissue.
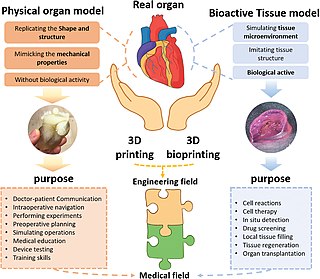
Three dimensional (3D) bioprinting is the utilization of 3D printing–like techniques to combine cells, growth factors, and/or biomaterials to fabricate biomedical parts, often with the aim of imitating natural tissue characteristics. Generally, 3D bioprinting can utilize a layer-by-layer method to deposit materials known as bio-inks to create tissue-like structures that are later used in various medical and tissue engineering fields. 3D bioprinting covers a broad range of bioprinting techniques and biomaterials. Currently, bioprinting can be used to print tissue and organ models to help research drugs and potential treatments. Nonetheless, translation of bioprinted living cellular constructs into clinical application is met with several issues due to the complexity and cell number needed to create functional organs. However, innovations span from bioprinting of extracellular matrix to mixing cells with hydrogels deposited layer by layer to produce the desired tissue. In addition, 3D bioprinting has begun to incorporate the printing of scaffolds. These scaffolds can be used to regenerate joints and ligaments.
Self-healing hydrogels are a specialized type of polymer hydrogel. A hydrogel is a macromolecular polymer gel constructed of a network of crosslinked polymer chains. Hydrogels are synthesized from hydrophilic monomers by either chain or step growth, along with a functional crosslinker to promote network formation. A net-like structure along with void imperfections enhance the hydrogel's ability to absorb large amounts of water via hydrogen bonding. As a result, hydrogels, self-healing alike, develop characteristic firm yet elastic mechanical properties. Self-healing refers to the spontaneous formation of new bonds when old bonds are broken within a material. The structure of the hydrogel along with electrostatic attraction forces drive new bond formation through reconstructive covalent dangling side chain or non-covalent hydrogen bonding. These flesh-like properties have motivated the research and development of self-healing hydrogels in fields such as reconstructive tissue engineering as scaffolding, as well as use in passive and preventive applications.
The in vivo bioreactor is a tissue engineering paradigm that uses bioreactor methodology to grow neotissue in vivo that augments or replaces malfunctioning native tissue. Tissue engineering principles are used to construct a confined, artificial bioreactor space in vivo that hosts a tissue scaffold and key biomolecules necessary for neotissue growth. Said space often requires inoculation with pluripotent or specific stem cells to encourage initial growth, and access to a blood source. A blood source allows for recruitment of stem cells from the body alongside nutrient delivery for continual growth. This delivery of cells and nutrients to the bioreactor eventually results in the formation of a neotissue product.
Platelet-rich fibrin (PRF) or leukocyte- and platelet-rich fibrin (L-PRF) is a derivative of PRP where autologous platelets and leukocytes are present in a complex fibrin matrix to accelerate the healing of soft and hard tissue and is used as a tissue-engineering scaffold for endodontics. PRF falls under FDA Product Code KST, labeling it as a blood draw/Hematology product classifying it as 510(k) exempt.
Bio-inks are materials used to produce engineered/artificial live tissue using 3D printing. These inks are mostly composed of the cells that are being used, but are often used in tandem with additional materials that envelope the cells. The combination of cells and usually biopolymer gels are defined as a bio-ink. They must meet certain characteristics, including such as rheological, mechanical, biofunctional and biocompatibility properties, among others. Using bio-inks provides a high reproducibility and precise control over the fabricated constructs in an automated manner. These inks are considered as one of the most advanced tools for tissue engineering and regenerative medicine (TERM).

Antonios Georgios Mikos is a Greek-American biomedical engineer who is the Louis Calder Professor of Bioengineering and Chemical and Biomolecular Engineering at Rice University. He specialises in biomaterials, drug delivery, and tissue engineering.
Nasal chondrocytes (NC) are present in the hyaline cartilage of the nasal septum and in fact are the only cell type within the tissue. Similar to chondrocytes present in articular cartilage, NC express extracellular matrix proteins such as glycosaminoglycans and collagen.
Tissue engineered heart valves (TEHV) offer a new and advancing proposed treatment of creating a living heart valve for people who are in need of either a full or partial heart valve replacement. Currently, there are over a quarter of a million prosthetic heart valves implanted annually, and the number of patients requiring replacement surgeries is only suspected to rise and even triple over the next fifty years. While current treatments offered such as mechanical valves or biological valves are not deleterious to one's health, they both have their own limitations in that mechanical valves necessitate the lifelong use of anticoagulants while biological valves are susceptible to structural degradation and reoperation. Thus, in situ (in its original position or place) tissue engineering of heart valves serves as a novel approach that explores the use creating a living heart valve composed of the host's own cells that is capable of growing, adapting, and interacting within the human body's biological system.
References
- ↑ Fibrin Sealants - test, blood, complications, time, infection, risk, rate, Definition, Purpose, Description, Preparation, Normal results
- ↑ Atrah HI (April 1994). "Fibrin glue". BMJ. 308 (6934): 933–4. doi:10.1136/bmj.308.6934.933. PMC 2539755 . PMID 8173397.
- ↑ Evans LA, Ferguson KH, Foley JP, Rozanski TA, Morey AF (April 2003). "Fibrin sealant for the management of genitourinary injuries, fistulas and surgical complications". The Journal of Urology. 169 (4): 1360–2. doi:10.1097/01.ju.0000052663.84060.ea. PMID 12629361.
- ↑ Feinstein AJ, Varela JE, Cohn SM, Compton RP, McKenney MG (2001). "Fibrin glue eliminates the need for packing after complex liver injuries". Yale Journal of Biology and Medicine . 74 (5): 315–21. PMC 2588746 . PMID 11769337.
- ↑ Bastarache JA (March 2009). "The complex role of fibrin in acute lung injury". American Journal of Physiology. Lung Cellular and Molecular Physiology. 296 (3): L275–6. doi:10.1152/ajplung.90633.2008. PMID 19118088.
- ↑ Modi P, Rahamim J (July 2005). "Fibrin sealant treatment of splenic injuries during oesophagectomy". European Journal of Cardio-Thoracic Surgery. 28 (1): 167–8. doi: 10.1016/j.ejcts.2005.02.045 . PMID 15876541.
- ↑ Patel R, Caruso RP, Taneja S, Stifelman M (November 2003). "Use of fibrin glue and gelfoam to repair collecting system injuries in a porcine model: implications for the technique of laparoscopic partial nephrectomy". Journal of Endourology. 17 (9): 799–804. doi:10.1089/089277903770802416. PMID 14642047.
- ↑ Toda K, Yoshitatsu M, Izutani H, Ihara K (August 2007). "Surgical management of penetrating cardiac injuries using a fibrin glue sheet". Interactive Cardiovascular and Thoracic Surgery. 6 (4): 577–8. doi: 10.1510/icvts.2007.156372 . PMID 17669945.
- ↑ "AME: Heart Valves". www.ame.hia.rwth-aachen.de. Retrieved 2010-05-31.
- ↑ "AME: Vascular Grafts". www.ame.hia.rwth-aachen.de. Retrieved 2010-05-31.
- ↑ Ahmed TA, Dare EV, Hincke M (June 2008). "Fibrin: a versatile scaffold for tissue engineering applications". Tissue Engineering Part B: Reviews. 14 (2): 199–215. doi:10.1089/ten.teb.2007.0435. PMID 18544016.
- 1 2 3 Uibo R, Laidmäe I, Sawyer ES, et al. (May 2009). "Soft materials to treat central nervous system injuries: evaluation of the suitability of non-mammalian fibrin gels". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1793 (5): 924–30. doi:10.1016/j.bbamcr.2009.01.007. PMC 2895977 . PMID 19344675.
- ↑ Shaikh FM, Callanan A, Kavanagh EG, Burke PE, Grace PA, McGloughlin TM (2008). "Fibrin: a natural biodegradable scaffold in vascular tissue engineering". Cells Tissues Organs. 188 (4): 333–46. doi:10.1159/000139772. PMID 18552484. S2CID 20286786.
- 1 2 3 4 Ye Q, Zünd G, Benedikt P, et al. (May 2000). "Fibrin gel as a three dimensional matrix in cardiovascular tissue engineering". European Journal of Cardio-Thoracic Surgery. 17 (5): 587–91. doi: 10.1016/S1010-7940(00)00373-0 . PMID 10814924.
- 1 2 Bensaïd W, Triffitt JT, Blanchat C, Oudina K, Sedel L, Petite H (June 2003). "A biodegradable fibrin scaffold for mesenchymal stem cell transplantation". Biomaterials. 24 (14): 2497–502. doi:10.1016/S0142-9612(02)00618-X. PMID 12695076.
- 1 2 Wozniak G (August 2003). "Fibrin sealants in supporting surgical techniques: The importance of individual components". Cardiovascular Surgery. 11 (Suppl 1): 17–21. doi:10.1016/S0967-2109(03)00067-X. PMID 12869984.
- ↑ Cholewinski E, Dietrich M, Flanagan TC, Schmitz-Rode T, Jockenhoevel S (November 2009). "Tranexamic acid--an alternative to aprotinin in fibrin-based cardiovascular tissue engineering". Tissue Engineering. Part A. 15 (11): 3645–53. CiteSeerX 10.1.1.527.8956 . doi:10.1089/ten.TEA.2009.0235. PMID 19496679.
- 1 2 Mol A, van Lieshout MI, Dam-de Veen CG, et al. (June 2005). "Fibrin as a cell carrier in cardiovascular tissue engineering applications". Biomaterials. 26 (16): 3113–21. doi:10.1016/j.biomaterials.2004.08.007. PMID 15603806.
- ↑ Aper T, Schmidt A, Duchrow M, Bruch HP (January 2007). "Autologous blood vessels engineered from peripheral blood sample". European Journal of Vascular and Endovascular Surgery. 33 (1): 33–9. doi: 10.1016/j.ejvs.2006.08.008 . PMID 17070080.
- ↑ Jockenhoevel S, Chalabi K, Sachweh JS, et al. (October 2001). "Tissue engineering: complete autologous valve conduit--a new moulding technique". The Thoracic and Cardiovascular Surgeon. 49 (5): 287–90. doi:10.1055/s-2001-17807. PMID 11605139.
- 1 2 Rowe SL, Lee S, Stegemann JP (January 2007). "Influence of thrombin concentration on the mechanical and morphological properties of cell-seeded fibrin hydrogels". Acta Biomaterialia. 3 (1): 59–67. doi:10.1016/j.actbio.2006.08.006. PMC 1852453 . PMID 17085089.
- ↑ Aper T, Teebken OE, Steinhoff G, Haverich A (September 2004). "Use of a fibrin preparation in the engineering of a vascular graft model". European Journal of Vascular and Endovascular Surgery. 28 (3): 296–302. doi: 10.1016/j.ejvs.2004.05.016 . PMID 15288634.
- ↑ Eyrich D, Brandl F, Appel B, et al. (January 2007). "Long-term stable fibrin gels for cartilage engineering". Biomaterials. 28 (1): 55–65. doi:10.1016/j.biomaterials.2006.08.027. PMID 16962167.
- ↑ Kjaergard HK, Weis-Fogh US (1994). "Important factors influencing the strength of autologous fibrin glue; the fibrin concentration and reaction time--comparison of strength with commercial fibrin glue". European Surgical Research. 26 (5): 273–6. doi:10.1159/000129346. PMID 7835384.
- ↑ Zhao H, Ma L, Zhou J, Mao Z, Gao C, Shen J (March 2008). "Fabrication and physical and biological properties of fibrin gel derived from human plasma". Biomedical Materials. 3 (1): 015001. Bibcode:2008BioMa...3a5001Z. doi:10.1088/1748-6041/3/1/015001. PMID 18458488.
- 1 2 Schense JC, Hubbell JA (1999). "Cross-linking exogenous bifunctional peptides into fibrin gels with factor XIIIa". Bioconjugate Chemistry. 10 (1): 75–81. doi:10.1021/bc9800769. PMID 9893967.
- ↑ Schense JC, Bloch J, Aebischer P, Hubbell JA (April 2000). "Enzymatic incorporation of bioactive peptides into fibrin matrices enhances neurite extension". Nature Biotechnology. 18 (4): 415–9. doi:10.1038/74473. PMID 10748522. S2CID 28502051.
- 1 2 Sakiyama-Elbert SE, Hubbell JA (April 2000). "Development of fibrin derivatives for controlled release of heparin-binding growth factors". Journal of Controlled Release. 65 (3): 389–402. doi:10.1016/S0168-3659(99)00221-7. PMID 10699297. S2CID 40929020.
- 1 2 Lee, A.C., et al., Experimental Neurology, 2003. 184(1): p. 295-303.
- ↑ Taylor SJ, McDonald JW, Sakiyama-Elbert SE (August 2004). "Controlled release of neurotrophin-3 from fibrin gels for spinal cord injury". Journal of Controlled Release. 98 (2): 281–94. doi:10.1016/j.jconrel.2004.05.003. PMID 15262419.
- ↑ Sakiyama-Elbert SE, Hubbell JA (October 2000). "Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix". Journal of Controlled Release. 69 (1): 149–58. doi:10.1016/S0168-3659(00)00296-0. PMID 11018553.
- ↑ Lyon M, Rushton G, Gallagher JT (July 1997). "The interaction of the transforming growth factor-betas with heparin/heparan sulfate is isoform-specific". The Journal of Biological Chemistry. 272 (29): 18000–6. doi: 10.1074/jbc.272.29.18000 . PMID 9218427.
- ↑ Appelman TP, Mizrahi J, Elisseeff JH, Seliktar D (February 2009). "The differential effect of scaffold composition and architecture on chondrocyte response to mechanical stimulation". Biomaterials. 30 (4): 518–25. doi:10.1016/j.biomaterials.2008.09.063. PMID 19000634.
- ↑ Yasuda H, Kuroda S, Shichinohe H, Kamei S, Kawamura R, Iwasaki Y (February 2010). "Effect of biodegradable fibrin scaffold on survival, migration, and differentiation of transplanted bone marrow stromal cells after cortical injury in rats". Journal of Neurosurgery. 112 (2): 336–44. doi:10.3171/2009.2.JNS08495. PMID 19267524.
- ↑ Zhu SJ, Choi BH, Huh JY, Jung JH, Kim BY, Lee SH (February 2006). "A comparative qualitative histological analysis of tissue-engineered bone using bone marrow mesenchymal stem cells, alveolar bone cells, and periosteal cells". Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics. 101 (2): 164–9. doi:10.1016/j.tripleo.2005.04.006. PMID 16448916.
- ↑ Altmeppen J, Hansen E, Bonnländer GL, Horch RE, Jeschke MG (April 2004). "Composition and characteristics of an autologous thrombocyte gel". The Journal of Surgical Research. 117 (2): 202–7. doi:10.1016/j.jss.2003.10.019. PMID 15047124.
- ↑ Le Nihouannen D, Guehennec LL, Rouillon T, et al. (May 2006). "Micro-architecture of calcium phosphate granules and fibrin glue composites for bone tissue engineering". Biomaterials. 27 (13): 2716–22. doi:10.1016/j.biomaterials.2005.11.038. PMID 16378638.
- ↑ Schmidt D, Hoerstrup SP (September 2006). "Tissue engineered heart valves based on human cells". Swiss Medical Weekly. 136 (39–40): 618–23. PMID 17086507.
- ↑ Flanagan TC, Sachweh JS, Frese J, et al. (October 2009). "In vivo remodeling and structural characterization of fibrin-based tissue-engineered heart valves in the adult sheep model". Tissue Engineering. Part A. 15 (10): 2965–76. doi:10.1089/ten.TEA.2009.0018. PMID 19320544.
- ↑ AME: Bioreactor Technologies
- ↑ Tschoeke B, Flanagan TC, Koch S, et al. (August 2009). "Tissue-engineered small-caliber vascular graft based on a novel biodegradable composite fibrin-polylactide scaffold". Tissue Engineering. Part A. 15 (8): 1909–18. doi:10.1089/ten.tea.2008.0499. PMID 19125650.
- ↑ Flanagan TC, Tschoeke B, Diamantouros S, Schmitz-Rode T, Jockenhoevel S (February 2009). "Mechanical properties of tissue-engineered vascular grafts: response to letter to the editor". Artificial Organs. 33 (2): 194–6. doi:10.1111/j.1525-1594.2008.00708.x. PMID 19178467.
- ↑ Koch S, Flanagan TC, Sachweh JS, et al. (June 2010). "Fibrin-polylactide-based tissue-engineered vascular graft in the arterial circulation". Biomaterials. 31 (17): 4731–9. doi:10.1016/j.biomaterials.2010.02.051. PMID 20304484.
- ↑ Gonçalves ED, Campos M, Paris F, Gomes JA, Farias CC (2008). "Ceratopatia bolhosa: etiopatogênese e tratamento" [Bullous keratopathy: etiopathogenesis and treatment]. Arquivos Brasileiros de Oftalmologia (in Portuguese). 71 (6 Suppl): 61–4. doi: 10.1590/S0004-27492008000700012 . PMID 19274413.
- ↑ Chawla B, Tandon R (2008). "Sutureless amniotic membrane fixation with fibrin glue in symptomatic bullous keratopathy with poor visual potential". European Journal of Ophthalmology. 18 (6): 998–1001. doi:10.1177/112067210801800623. PMID 18988175. S2CID 36302246.
- ↑ Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA (April 2006). "Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures". Biophysical Journal. 90 (8): 3012–8. Bibcode:2006BpJ....90.3012G. doi:10.1529/biophysj.105.073114. PMC 1414567 . PMID 16461391.
- ↑ Saha K, Keung AJ, Irwin EF, et al. (November 2008). "Substrate modulus directs neural stem cell behavior". Biophysical Journal. 95 (9): 4426–38. Bibcode:2008BpJ....95.4426S. doi:10.1529/biophysj.108.132217. PMC 2567955 . PMID 18658232.
- ↑ Ju YE, Janmey PA, McCormick ME, Sawyer ES, Flanagan LA (April 2007). "Enhanced neurite growth from mammalian neurons in three-dimensional salmon fibrin gels". Biomaterials. 28 (12): 2097–108. doi:10.1016/j.biomaterials.2007.01.008. PMC 1991290 . PMID 17258313.
- ↑ Wood MD, Moore AM, Hunter DA, et al. (May 2009). "Affinity-based release of glial-derived neurotrophic factor from fibrin matrices enhances sciatic nerve regeneration". Acta Biomaterialia. 5 (4): 959–68. doi:10.1016/j.actbio.2008.11.008. PMC 2678870 . PMID 19103514.
- ↑ Lei P, Padmashali RM, Andreadis ST (August 2009). "Cell-controlled and spatially arrayed gene delivery from fibrin hydrogels". Biomaterials. 30 (22): 3790–9. doi:10.1016/j.biomaterials.2009.03.049. PMC 2692826 . PMID 19395019.