Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It potentially results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The mechanism of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin.

Disseminated intravascular coagulation (DIC) is a condition in which blood clots form throughout the body, blocking small blood vessels. Symptoms may include chest pain, shortness of breath, leg pain, problems speaking, or problems moving parts of the body. As clotting factors and platelets are used up, bleeding may occur. This may include blood in the urine, blood in the stool, or bleeding into the skin. Complications may include organ failure.

Fibrin is a fibrous, non-globular protein involved in the clotting of blood. It is formed by the action of the protease thrombin on fibrinogen, which causes it to polymerize. The polymerized fibrin, together with platelets, forms a hemostatic plug or clot over a wound site.

Fibrinogen is a glycoprotein complex, produced in the liver, that circulates in the blood of all vertebrates. During tissue and vascular injury, it is converted enzymatically by thrombin to fibrin and then to a fibrin-based blood clot. Fibrin clots function primarily to occlude blood vessels to stop bleeding. Fibrin also binds and reduces the activity of thrombin. This activity, sometimes referred to as antithrombin I, limits clotting. Fibrin also mediates blood platelet and endothelial cell spreading, tissue fibroblast proliferation, capillary tube formation, and angiogenesis and thereby promotes revascularization and wound healing.

Thrombin is a serine protease, an enzyme that, in humans, is encoded by the F2 gene.
Fibrinolysis is a process that prevents blood clots from growing and becoming problematic. Primary fibrinolysis is a normal body process, while secondary fibrinolysis is the breakdown of clots due to a medicine, a medical disorder, or some other cause.
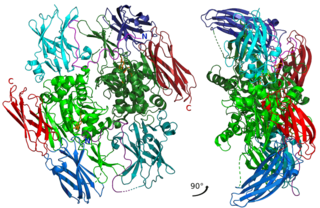
Factor XIII or fibrin stabilizing factor is a zymogen found in blood of humans and some other animals. It is activated by thrombin to factor XIIIa. Factor XIIIa is an enzyme of the blood coagulation system that crosslinks fibrin. Deficiency of XIII worsens clot stability and increases bleeding tendency.

The thrombin time (TT), also known as the thrombin clotting time (TCT), is a blood test that measures the time it takes for a clot to form in the plasma of a blood sample containing anticoagulant, after an excess of thrombin has been added. It is used to diagnose blood coagulation disorders and to assess the effectiveness of fibrinolytic therapy. This test is repeated with pooled plasma from normal patients. The difference in time between the test and the 'normal' indicates an abnormality in the conversion of fibrinogen to fibrin, an insoluble protein.
The Cohn process, developed by Edwin J. Cohn, is a series of purification steps with the purpose of extracting albumin from blood plasma. The process is based on the differential solubility of albumin and other plasma proteins based on pH, ethanol concentration, temperature, ionic strength, and protein concentration. Albumin has the highest solubility and lowest isoelectric point of all the major plasma proteins. This makes it the final product to be precipitated, or separated from its solution in a solid form. Albumin was an excellent substitute for human plasma in World War Two. When administered to wounded soldiers or other patients with blood loss, it helped expand the volume of blood and led to speedier recovery. Cohn's method was gentle enough that isolated albumin protein retained its biological activity.

Batroxobin, also known as reptilase, is a snake venom enzyme with Venombin A activity produced by Bothrops atrox and Bothrops moojeni, venomous species of pit viper found east of the Andes in South America. It is a hemotoxin which acts as a serine protease similarly to thrombin, and has been the subject of many medical studies as a replacement of thrombin. Different enzymes, isolated from different species of Bothrops, have been called batroxobin, but unless stated otherwise, this article covers the batroxobin produced by B. moojeni, as this is the most studied variety.
Tachosil is an equine collagen sponge coated with the human plasma-derived coagulation factors fibrinogen and thrombin. It is used during surgery to stop local bleeding on internal organs (hemostasis). Tachosil reacts upon contact with blood, other body fluids or saline to form a clot that glues it to the tissue surface.
The dysfibrinogenemias consist of three types of fibrinogen disorders in which a critical blood clotting factor, fibrinogen, circulates at normal levels but is dysfunctional. Congenital dysfibrinogenemia is an inherited disorder in which one of the parental genes produces an abnormal fibrinogen. This fibrinogen interferes with normal blood clotting and/or lysis of blood clots. The condition therefore may cause pathological bleeding and/or thrombosis. Acquired dysfibrinogenemia is a non-hereditary disorder in which fibrinogen is dysfunctional due to the presence of liver disease, autoimmune disease, a plasma cell dyscrasias, or certain cancers. It is associated primarily with pathological bleeding. Hereditary fibrinogen Aα-Chain amyloidosis is a sub-category of congenital dysfibrinogenemia in which the dysfunctional fibrinogen does not cause bleeding or thrombosis but rather gradually accumulates in, and disrupts the function of, the kidney.

Fibrinogen alpha chain is a protein that in humans is encoded by the FGA gene.

Fibrinogen beta chain, also known as FGB, is a gene found in humans and most other vertebrates with a similar system of blood coagulation.
The fibrinolysis system is responsible for removing blood clots. Hyperfibrinolysis describes a situation with markedly enhanced fibrinolytic activity, resulting in increased, sometimes catastrophic bleeding. Hyperfibrinolysis can be caused by acquired or congenital reasons. Among the congenital conditions for hyperfibrinolysis, deficiency of alpha-2-antiplasmin or plasminogen activator inhibitor type 1 (PAI-1) are very rare. The affected individuals show a hemophilia-like bleeding phenotype. Acquired hyperfibrinolysis is found in liver disease, in patients with severe trauma, during major surgical procedures, and other conditions. A special situation with temporarily enhanced fibrinolysis is thrombolytic therapy with drugs which activate plasminogen, e.g. for use in acute ischemic events or in patients with stroke. In patients with severe trauma, hyperfibrinolysis is associated with poor outcome. Moreover, hyperfibrinolysis may be associated with blood brain barrier impairment, a plasmin-dependent effect due to an increased generation of bradykinin.
A fibrin scaffold is a network of protein that holds together and supports a variety of living tissues. It is produced naturally by the body after injury, but also can be engineered as a tissue substitute to speed healing. The scaffold consists of naturally occurring biomaterials composed of a cross-linked fibrin network and has a broad use in biomedical applications.
Platelet-Poor Plasma (PPP) is blood plasma with very low number of platelets (< 10 X 103/μL). Traditionally, PPP was recommended for use in platelet aggregation studies to both adjust the platelet-rich plasma concentration, and to serve as a control. PPP may have elevated levels of fibrinogen, which has the ability to form a fibrin-rich clot once activated. Wound healing requires cell migration and attachment, which is facilitated by this fibrin clot.
Blood clotting tests are the tests used for diagnostics of the hemostasis system. Coagulometer is the medical laboratory analyzer used for testing of the hemostasis system. Modern coagulometers realize different methods of activation and observation of development of blood clots in blood or in blood plasma.
Congenital hypofibrinogenemia is a rare disorder in which one of the three genes responsible for producing fibrinogen, a critical blood clotting factor, is unable to make a functional fibrinogen glycoprotein because of an inherited mutation. In consequence, liver cells, the normal site of fibrinogen production, make small amounts of this critical coagulation protein, blood levels of fibrinogen are low, and individuals with the disorder may develop a coagulopathy, i.e. a diathesis or propensity to experience episodes of abnormal bleeding. However, individuals with congenital hypofibrinogenemia may also have episodes of abnormal blood clot formation, i.e. thrombosis. This seemingly paradoxical propensity to develop thrombosis in a disorder causing a decrease in a critical protein for blood clotting may be due to the function of fibrin to promote the lysis or disintegration of blood clots. Lower levels of fibrin may reduce the lysis of early fibrin strand depositions and thereby allow these depositions to develop into clots.
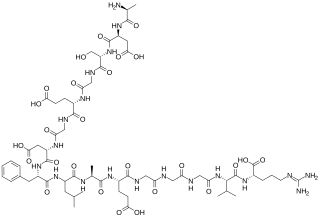
The fibrinopeptides, fibrinopeptide A (FpA) and fibrinopeptide B (FpB), are peptides which are located in the central region of the fibrous glycoprotein fibrinogen and are cleaved by the enzyme thrombin to convert fibrinogen into covalently-linked fibrin monomers. The N-terminal FpA is cleaved from the Aα chains of fibrinogen and FpB from the Bβ chains of fibrinogen, with FpA released before FpB. Subsequent to their formation, fibrin monomers are converted to cross-linked fibrin polymers by the action of thrombin-activated factor XIII, and these fibrin polymers form the backbone of a thrombus. Hence, the fibrinopeptides are sensitive markers of fibrinogenesis, thrombin activity, and coagulation.