Related Research Articles

Factor VIII is a medication used to treat and prevent bleeding in people with hemophilia A and other causes of low factor VIII. Certain preparations may also be used in those with von Willebrand's disease. It is given by slow injection into a vein.
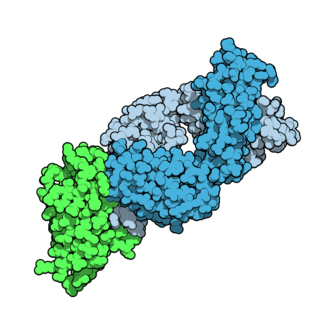
Tremelimumab, sold under the brand name Imjudo, is a fully human monoclonal antibody used for the treatment of hepatocellular carcinoma. Tremelimumab is designed to attach to and block CTLA-4, a protein that controls the activity of T cells, which are part of the immune system.

Insulin aspart, sold under the brand name NovoLog, among others, is a modified type of medical insulin used to treat type 1 and type 2 diabetes. It is generally used by injection under the skin but may also be used by injection into a vein. Maximum effect occurs after about 1–3 hours and lasts for 3–5 hours. Generally a longer-acting insulin like insulin NPH is also needed.

Linagliptin, sold under the brand name Tradjenta among others, is a medication used to treat type 2 diabetes in conjunction with exercise and diet. It is generally less preferred than metformin and sulfonylureas as an initial treatment. It is taken by mouth.
Turoctocog alfa is a recombinant antihemophilic factor VIII used for the treatment of and prophylaxis of bleeding patients with haemophilia A. It is marketed by Novo Nordisk. It was approved in the United States, the European Union, and Japan in 2013.

Sacituzumab govitecan, sold under the brand name Trodelvy by Gilead Sciences, is a Trop-2-directed antibody and topoisomerase inhibitor drug conjugate used for the treatment of metastatic triple-negative breast cancer and metastatic urothelial cancer.

Osimertinib, sold under the brand name Tagrisso, is a medication used to treat non-small-cell lung carcinomas with specific mutations. It is a third-generation epidermal growth factor receptor tyrosine kinase inhibitor.

Semaglutide is an antidiabetic medication used for the treatment of type 2 diabetes and an anti-obesity medication used for long-term weight management. It is a peptide similar to the hormone glucagon-like peptide-1 (GLP-1), modified with a side chain. It can be administered by subcutaneous injection or taken orally. It is sold under the brand names Ozempic and Rybelsus for diabetes, and under the brand name Wegovy for weight loss.
Burosumab, sold under the brand name Crysvita, is a human monoclonal antibody medication approved 2018 for the treatment of X-linked hypophosphatemia and tumor-induced osteomalacia.
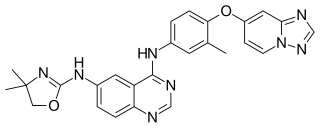
Tucatinib, sold under the brand name Tukysa, is an anticancer medication used for the treatment of HER2-positive breast cancer. It is a small molecule inhibitor of HER2. It was developed by Array BioPharma and licensed to Cascadian Therapeutics.
Cenegermin, sold under the brand name Oxervate, also known as recombinant human nerve growth factor (rhNGF), is a recombinant form of human nerve growth factor (NGF). In July 2017, it was approved in the European Union as an eye drop formulation for the treatment of moderate or severe neurotrophic keratitis in adults.
Ravulizumab, sold under the brand name Ultomiris, is a humanized monoclonal antibody complement inhibitor medication designed for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome. It is designed to bind to and prevent the activation of Complement component 5 (C5).
Bimekizumab, sold under the brand name Bimzelx, is a humanized anti-IL17A, anti-IL-17F, and anti-IL17AF monoclonal antibody that is used to treat plaque psoriasis, psoriatic arthritis, axial spondyloarthritis, and ankylosing spondylitis.

Pemigatinib, sold under the brand name Pemazyre, is an anti-cancer medication used for the treatment of bile duct cancer (cholangiocarcinoma). Pemigatinib works by blocking FGFR2 in tumor cells to prevent them from growing and spreading.
Tafasitamab, sold under the brand name Monjuvi, is a medication used in combination with lenalidomide for the treatment of adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL).
Somatrogon, sold under the brand name Ngenla, is a medication for the treatment of growth hormone deficiency. Somatrogon is a glycosylated protein constructed from human growth hormone and a small part of human chorionic gonadotropin which is appended to both the N-terminal and C-terminal. Somatrogon is a human growth hormone analog.
Ciltacabtagene autoleucel, sold under the brand name Carvykti, is an anti-cancer medication used to treat multiple myeloma. Ciltacabtagene autoleucel is a BCMA -directed genetically modified autologous chimeric antigen receptor (CAR) T-cell therapy. Each dose is customized using the recipient's own T-cells, which are collected and genetically modified, and infused back into the recipient.

Mavacamten, sold under the brand name Camzyos, is a medication used to treat obstructive hypertrophic cardiomyopathy.
Teclistamab, sold under the brand name Tecvayli, is a human bispecific monoclonal antibody used for the treatment of relapsed and refractory multiple myeloma. It is a bispecific antibody that targets the CD3 receptor expressed on the surface of T-cells and B-cell maturation antigen (BCMA), which is expressed on the surface of malignant multiple myeloma B-lineage cells.

Deucravacitinib, sold under the brand name Sotyktu, is a medication used for the treatment of moderate-to-severe plaque psoriasis. It is a tyrosine kinase 2 (TYK2) inhibitor and it is taken by mouth. It was developed by Bristol Myers Squibb.
References
- 1 2 "Alhemo APMDS". Therapeutic Goods Administration (TGA). 29 September 2023. Archived from the original on 10 March 2024. Retrieved 7 March 2024.
- ↑ "Alhemo (Novo Nordisk Pharmaceuticals Pty Ltd)". Therapeutic Goods Administration (TGA). 28 July 2023. Archived from the original on 10 March 2024. Retrieved 10 September 2023.
- ↑ "AusPAR: Alhemo". Therapeutic Goods Administration (TGA). 18 March 2024. Retrieved 31 March 2024.
- 1 2 "Alhemo Product information". Health Canada. 10 March 2023. Archived from the original on 24 March 2023. Retrieved 9 June 2023.
- 1 2 3 4 "Alhemo (concizumab injection) Product Monograph" (PDF). Health Canada. Archived (PDF) from the original on 3 March 2024. Retrieved 3 March 2024.
- 1 2 "Summary Basis of Decision for Alhemo". Health Canada . 31 May 2023. Archived from the original on 10 March 2024. Retrieved 9 June 2023.
- ↑ "Health Canada approves Alhemo, the first subcutaneous prophylactic treatment for people living with hemophilia B with inhibitors" (Press release). Novo Nordisk Canada. 17 April 2023. Archived from the original on 9 June 2023. Retrieved 9 June 2023– via Newswire.
- ↑ World Health Organization (2013). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 70". WHO Drug Information. 27 (3). hdl: 10665/331167 .