
Anticoagulants, commonly known as blood thinners, are chemical substances that prevent or reduce coagulation of blood, prolonging the clotting time. Some of them occur naturally in blood-eating animals such as leeches and mosquitoes, where they help keep the bite area unclotted long enough for the animal to obtain some blood. As a class of medications, anticoagulants are used in therapy for thrombotic disorders. Oral anticoagulants (OACs) are taken by many people in pill or tablet form, and various intravenous anticoagulant dosage forms are used in hospitals. Some anticoagulants are used in medical equipment, such as sample tubes, blood transfusion bags, heart–lung machines, and dialysis equipment. One of the first anticoagulants, warfarin, was initially approved as a rodenticide.

Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It potentially results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The mechanism of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin.

Warfarin is an anticoagulant used as a medication under several brand names including Coumadin. While the drug is described as a "blood thinner", it does not reduce viscosity but rather inhibits coagulation. Accordingly, it is commonly used to prevent blood clots in the circulatory system such as deep vein thrombosis and pulmonary embolism, and to protect against stroke in people who have atrial fibrillation, valvular heart disease, or artificial heart valves. Less commonly, it is used following ST-segment elevation myocardial infarction and orthopedic surgery. It is usually taken by mouth, but may also be administered intravenously.

Thrombin is a serine protease, an enzyme that, in humans, is encoded by the F2 gene. Prothrombin is proteolytically cleaved to form thrombin in the clotting process. Thrombin in turn acts as a serine protease that converts soluble fibrinogen into insoluble strands of fibrin, as well as catalyzing many other coagulation-related reactions.
Low-molecular-weight heparin (LMWH) is a class of anticoagulant medications. They are used in the prevention of blood clots and treatment of venous thromboembolism and in the treatment of myocardial infarction.

The prothrombin time (PT) – along with its derived measures of prothrombin ratio (PR) and international normalized ratio (INR) – is an assay for evaluating the extrinsic pathway and common pathway of coagulation. This blood test is also called protime INR and PT/INR. They are used to determine the clotting tendency of blood, in such things as the measure of warfarin dosage, liver damage, and vitamin K status. PT measures the following coagulation factors: I (fibrinogen), II (prothrombin), V (proaccelerin), VII (proconvertin), and X.
Mixing studies are tests performed on blood plasma of patients or test subjects to distinguish factor deficiencies from factor inhibitors, such as lupus anticoagulant, or specific factor inhibitors, such as antibodies directed against factor VIII. The basic purpose of these tests is to determine the cause of prolongation of Prothrombin Time (PT), Partial Thromboplastin Time, or sometimes of thrombin time (TT). Mixing studies take advantage of the fact that factor levels that are 50 percent of normal should give a normal Prothrombin time (PT) or Partial thromboplastin time (PTT) result. Factor deficient plasmas are used in mixing studies. Plasma with known factor deficiencies are commercially available but are very expensive, so they are often prepared in the laboratory and can then be used for mixing experiments.

Factor X, also known by the eponym Stuart–Prower factor, is an enzyme of the coagulation cascade. It is a serine endopeptidase. Factor X is synthesized in the liver and requires vitamin K for its synthesis.
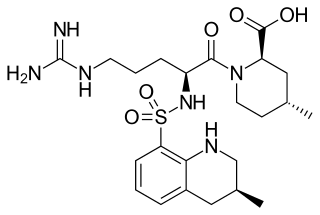
Argatroban is an anticoagulant that is a small molecule direct thrombin inhibitor. In 2000, argatroban was licensed by the Food and Drug Administration (FDA) for prophylaxis or treatment of thrombosis in patients with heparin-induced thrombocytopenia (HIT). In 2002, it was approved for use during percutaneous coronary interventions in patients who have HIT or are at risk for developing it. In 2012, it was approved by the MHRA in the UK for anticoagulation in patients with heparin-induced thrombocytopenia Type II (HIT) who require parenteral antithrombotic therapy.

Fresh frozen plasma (FFP) is a blood product made from the liquid portion of whole blood. It is used to treat conditions in which there are low blood clotting factors or low levels of other blood proteins. It may also be used as the replacement fluid in plasma exchange. Using ABO compatible plasma, while not required, may be recommended. Use as a volume expander is not recommended. It is given by slow injection into a vein.

Rivaroxaban, sold under the brand name Xarelto among others, is an anticoagulant medication used to treat and prevent blood clots. Specifically it is used to treat deep vein thrombosis and pulmonary emboli and prevent blood clots in atrial fibrillation and following hip or knee surgery. It is taken by mouth.

Warfarin-induced skin necrosis is a condition in which skin and subcutaneous tissue necrosis occurs due to acquired protein C deficiency following treatment with anti-vitamin K anticoagulants.

Dabigatran, sold under the brand name Pradaxa among others, is an anticoagulant used to treat and prevent blood clots and to prevent stroke in people with atrial fibrillation. Specifically it is used to prevent blood clots following hip or knee replacement and in those with a history of prior clots. It is used as an alternative to warfarin and does not require monitoring by blood tests. In a meta analysis of 7 different studies, there was no benefit of dabigatran over warfarin in preventing ischemic stroke; however, dabigatran were associated with a lower hazard for intracranial bleeding compared with warfarin, but also had a higher risk of gastrointestinal bleeding relative to warfarin. It is taken by mouth.
Hypercoagulability in pregnancy is the propensity of pregnant women to develop thrombosis. Pregnancy itself is a factor of hypercoagulability, as a physiologically adaptive mechanism to prevent post partum bleeding. However, when combined with an additional underlying hypercoagulable states, the risk of thrombosis or embolism may become substantial.
Direct factor Xa inhibitors (xabans) are anticoagulants, used to both treat and prevent blood clots in veins, and prevent stroke and embolism in people with atrial fibrillation (AF).
Thromboelastometry (TEM), previously named rotational thromboelastography (ROTEG) or rotational thromboelastometry (ROTEM), is an established viscoelastic method for hemostasis testing in whole blood. It is a modification of traditional thromboelastography (TEG).

Edoxaban, sold under the brand name Lixiana among others, is an anticoagulant medication and a direct factor Xa inhibitor. It is taken by mouth.

Apixaban, sold under the brand name Eliquis, is an anticoagulant medication used to treat and prevent blood clots and to prevent stroke in people with nonvalvular atrial fibrillation through directly inhibiting factor Xa. Specifically, it is used to prevent blood clots following hip or knee replacement and in those with a history of prior clots. It is used as an alternative to warfarin and does not require monitoring by blood tests or dietary restrictions. It is taken by mouth.
Direct thrombin inhibitors (DTIs) are a class of anticoagulant drugs that can be used to prevent and treat embolisms and blood clots caused by various diseases. They inhibit thrombin, a serine protease which affects the coagulation cascade in many ways. DTIs have undergone rapid development since the 90's. With technological advances in genetic engineering the production of recombinant hirudin was made possible which opened the door to this new group of drugs. Before the use of DTIs the therapy and prophylaxis for anticoagulation had stayed the same for over 50 years with the use of heparin derivatives and warfarin which have some well known disadvantages. DTIs are still under development, but the research focus has shifted towards factor Xa inhibitors, or even dual thrombin and fXa inhibitors that have a broader mechanism of action by both inhibiting factor IIa (thrombin) and Xa. A recent review of patents and literature on thrombin inhibitors has demonstrated that the development of allosteric and multi-mechanism inhibitors might lead the way to a safer anticoagulant.

Ciraparantag (aripazine) is a drug under investigation as an antidote for a number of anticoagulant drugs, including factor Xa inhibitors, dabigatran, and heparins.














