
Adenylate cyclase is an enzyme with systematic name ATP diphosphate-lyase . It catalyzes the following reaction:
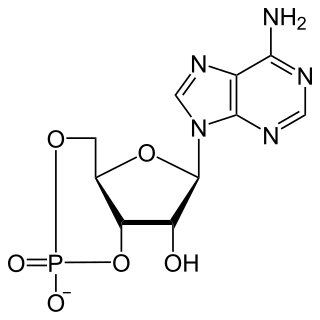
Cyclic adenosine monophosphate is a second messenger, or cellular signal occurring within cells, that is important in many biological processes. cAMP is a derivative of adenosine triphosphate (ATP) and used for intracellular signal transduction in many different organisms, conveying the cAMP-dependent pathway.

A cyclic nucleotide (cNMP) is a single-phosphate nucleotide with a cyclic bond arrangement between the sugar and phosphate groups. Like other nucleotides, cyclic nucleotides are composed of three functional groups: a sugar, a nitrogenous base, and a single phosphate group. As can be seen in the cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) images, the 'cyclic' portion consists of two bonds between the phosphate group and the 3' and 5' hydroxyl groups of the sugar, very often a ribose.

Guanylate cyclase is a lyase enzyme that converts guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP) and pyrophosphate:

Alfred Goodman Gilman was an American pharmacologist and biochemist. He and Martin Rodbell shared the 1994 Nobel Prize in Physiology or Medicine "for their discovery of G-proteins and the role of these proteins in signal transduction in cells."

Adenylyl cyclase type 6 is an enzyme that in humans is encoded by the ADCY6 gene.

Adenylyl cyclase type 3 is an enzyme that in humans is encoded by the ADCY3 gene.

Adenylyl cyclase type 5 is an enzyme that in humans is encoded by the ADCY5 gene.

Adenylyl cyclase type 1 is an enzyme that in humans is encoded by the ADCY1 gene.

Adenylyl cyclase type 2 is an enzyme typically expressed in the brain of humans, that is encoded by the ADCY2 gene. It belongs to the adenylyl cyclase class-3 or guanylyl cyclase family because it contains two guanylate cyclase domains. ADCY2 is one of ten different mammalian isoforms of adenylyl cyclases. ADCY2 can be found on chromosome 5 and the "MIR2113-POU3F2" region of chromosome 6, with a length of 1091 amino-acids. An essential cofactor for ADCY2 is magnesium; two ions bind per subunit.

Adenylyl cyclase type 7 is an enzyme that in humans is encoded by the ADCY7 gene.

Adenylyl cyclase type 9 is an enzyme that in humans is encoded by the ADCY9 gene.

Adenylyl cyclase type 8 is an enzyme that in humans is encoded by the ADCY8 gene.

Adenylyl cyclase type 4 is an enzyme that in humans is encoded by the ADCY4 gene.
In the field of molecular biology, the cAMP-dependent pathway, also known as the adenylyl cyclase pathway, is a G protein-coupled receptor-triggered signaling cascade used in cell communication.
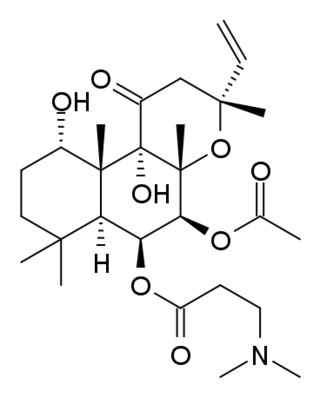
Colforsin daropate is a carboxylic ester derived from the condensation of forskolin (colforsin) with N,N-dimethyl-β-alanine.

Bithionol is an antibacterial, anthelmintic, and algaecide. It is used to treat Anoplocephala perfoliata (tapeworms) in horses and Fasciola hepatica.

Adenylyl cyclase 10 also known as ADCY10 is an enzyme that, in humans, is encoded by the ADCY10 gene.
In molecular biology, this protein domain belongs to the terpene synthase family (TPS). Its role is to synthesize terpenes, which are part of primary metabolism, such as sterols and carotene, and also part of the secondary metabolism. This entry will focus on the C terminal domain of the TPS protein.
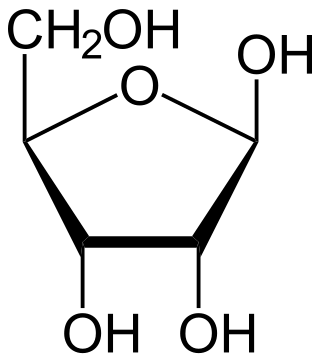
Ribose is a simple sugar and carbohydrate with molecular formula C5H10O5 and the linear-form composition H−(C=O)−(CHOH)4−H. The naturally-occurring form, d-ribose, is a component of the ribonucleotides from which RNA is built, and so this compound is necessary for coding, decoding, regulation and expression of genes. It has a structural analog, deoxyribose, which is a similarly essential component of DNA. l-ribose is an unnatural sugar that was first prepared by Emil Fischer and Oscar Piloty in 1891. It was not until 1909 that Phoebus Levene and Walter Jacobs recognised that d-ribose was a natural product, the enantiomer of Fischer and Piloty's product, and an essential component of nucleic acids. Fischer chose the name "ribose" as it is a partial rearrangement of the name of another sugar, arabinose, of which ribose is an epimer at the 2' carbon; both names also relate to gum arabic, from which arabinose was first isolated and from which they prepared l-ribose.



















