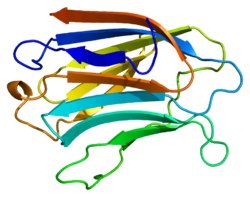
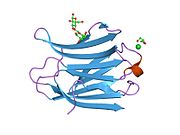
Fibronectin is a high-molecular weight glycoprotein of the extracellular matrix that binds to membrane-spanning receptor proteins called integrins. It is approved for marketing as a topical solution in India by Central Drugs Standard Control organization in 2020 under the brand name FIBREGA for chronic wounds. Fibronectin also binds to other extracellular matrix proteins such as collagen, fibrin, and heparan sulfate proteoglycans.

In biology, the extracellular matrix (ECM), also called intercellular matrix, is a network consisting of extracellular macromolecules and minerals, such as collagen, enzymes, glycoproteins and hydroxyapatite that provide structural and biochemical support to surrounding cells. Because multicellularity evolved independently in different multicellular lineages, the composition of ECM varies between multicellular structures; however, cell adhesion, cell-to-cell communication and differentiation are common functions of the ECM.

Cell adhesion is the process by which cells interact and attach to neighbouring cells through specialised molecules of the cell surface. This process can occur either through direct contact between cell surfaces such as cell junctions or indirect interaction, where cells attach to surrounding extracellular matrix, a gel-like structure containing molecules released by cells into spaces between them. Cells adhesion occurs from the interactions between cell-adhesion molecules (CAMs), transmembrane proteins located on the cell surface. Cell adhesion links cells in different ways and can be involved in signal transduction for cells to detect and respond to changes in the surroundings. Other cellular processes regulated by cell adhesion include cell migration and tissue development in multicellular organisms. Alterations in cell adhesion can disrupt important cellular processes and lead to a variety of diseases, including cancer and arthritis. Cell adhesion is also essential for infectious organisms, such as bacteria or viruses, to cause diseases.

Osteopontin (OPN), also known as bone /sialoprotein I, early T-lymphocyte activation (ETA-1), secreted phosphoprotein 1 (SPP1), 2ar and Rickettsia resistance (Ric), is a protein that in humans is encoded by the SPP1 gene. The murine ortholog is Spp1. Osteopontin is a SIBLING (glycoprotein) that was first identified in 1986 in osteoblasts.
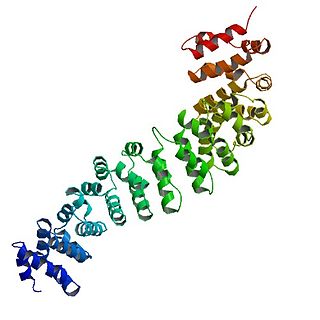
Catenin beta-1, also known as beta-catenin (β-catenin), is a protein that in humans is encoded by the CTNNB1 gene.

Mannan-binding lectin serine protease 1 also known as mannose-associated serine protease 1 (MASP-1) is an enzyme that in humans is encoded by the MASP1 gene.

Galectins are a class of proteins that bind specifically to β-galactoside sugars, such as N-acetyllactosamine, which can be bound to proteins by either N-linked or O-linked glycosylation. They are also termed S-type lectins due to their dependency on disulphide bonds for stability and carbohydrate binding. There have been about 15 galectins discovered in mammals, encoded by the LGALS genes, which are numbered in a consecutive manner. Only galectin-1, -2, -3, -4, -7, -7B, -8, -9, -9B, 9C, -10, -12, -13, -14, and -16 have been identified in humans. Galectin-5 and -6 are found in rodents, whereas galectin-11 and -15 are uniquely found in sheep and goats. Members of the galectin family have also been discovered in other mammals, birds, amphibians, fish, nematodes, sponges, and some fungi. Unlike the majority of lectins they are not membrane bound, but soluble proteins with both intra- and extracellular functions. They have distinct but overlapping distributions but found primarily in the cytosol, nucleus, extracellular matrix or in circulation. Although many galectins must be secreted, they do not have a typical signal peptide required for classical secretion. The mechanism and reason for this non-classical secretion pathway is unknown.

Galectin-1 is a protein that in humans is encoded by the LGALS1 gene.

Integrin beta-6 is a protein that in humans is encoded by the ITGB6 gene. It is the β6 subunit of the integrin αvβ6. Integrins are αβ heterodimeric glycoproteins which span the cell’s membrane, integrating the outside and inside of the cell. Integrins bind to specific extracellular proteins in the extracellular matrix or on other cells and subsequently transduce signals intracellularly to affect cell behaviour. One α and one β subunit associate non-covalently to form 24 unique integrins found in mammals. While some β integrin subunits partner with multiple α subunits, β6 associates exclusively with the αv subunit. Thus, the function of ITGB6 is entirely associated with the integrin αvβ6.

Galectin-3-binding protein is a protein that in humans is encoded by the LGALS3BP gene.

Galectin-10 is an enzyme that in humans is encoded by the CLC gene.

Galectin-8 is a protein of the galectin family that in humans is encoded by the LGALS8 gene.

Galectin-9 was first isolated from mouse embryonic kidney in 1997 as a 36 kDa beta-galactoside lectin protein. Human galectin-9 is encoded by the LGALS9 gene.

Galectin-2 is a protein that in humans is encoded by the LGALS2 gene.

Hepatitis A virus cellular receptor 2 (HAVCR2), also known as T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), is a protein that in humans is encoded by the HAVCR2 (TIM-3)gene. HAVCR2 was first described in 2002 as a cell surface molecule expressed on IFNγ producing CD4+ Th1 and CD8+ Tc1 cells. Later, the expression was detected in Th17 cells, regulatory T-cells, and innate immune cells. HAVCR2 receptor is a regulator of the immune response.

Galectin-7 is a protein that in humans is encoded by the LGALS7 gene.

Placental protein 13 (PP13) is a protein that in humans is encoded by the LGALS13 gene.
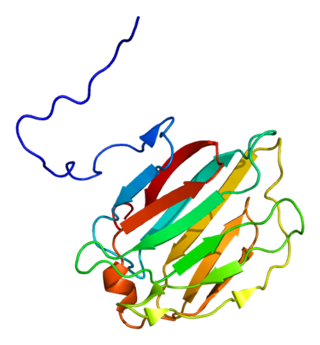
Galectin-4 is a protein that in humans is encoded by the LGALS4 gene.
Cenderitide is a natriuretic peptide developed by the Mayo Clinic as a potential treatment for heart failure. Cenderitide is created by the fusion of the 15 amino acid C-terminus of dendroaspis natriuretic peptide (DNP) with the full C-type natriuretic peptide (CNP) structure both peptide which are endogenous to humans. This peptide chimera is a dual activator of the natriuretic peptide receptors NPR-A and NPR-B and therefore exhibits the natriuretic and diuretic properties of DNP, as well as the antiproliferative and antifibrotic properties of CNP.
MFAP4 is an extracellular matrix protein encoded by the MFAP4 gene. It is part of the MFAP family of proteoglycans, which are involved in cell adhesion, intercellular interactions and the assembly and/or maintenance of elastic fibres.