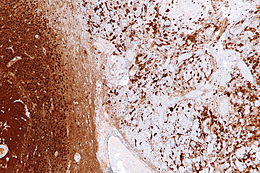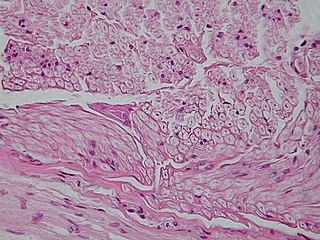
Nervous tissue, also called neural tissue, is the main tissue component of the nervous system. The nervous system regulates and controls body functions and activity. It consists of two parts: the central nervous system (CNS) comprising the brain and spinal cord, and the peripheral nervous system (PNS) comprising the branching peripheral nerves. It is composed of neurons, also known as nerve cells, which receive and transmit impulses, and neuroglia, also known as glial cells or glia, which assist the propagation of the nerve impulse as well as provide nutrients to the neurons.

Glia, also called glial cells(gliocytes) or neuroglia, are non-neuronal cells in the central nervous system (brain and spinal cord) and the peripheral nervous system that do not produce electrical impulses. The neuroglia make up more than one half the volume of neural tissue in our body. They maintain homeostasis, form myelin in the peripheral nervous system, and provide support and protection for neurons. In the central nervous system, glial cells include oligodendrocytes, astrocytes, ependymal cells and microglia, and in the peripheral nervous system they include Schwann cells and satellite cells.

Astrocytes, also known collectively as astroglia, are characteristic star-shaped glial cells in the brain and spinal cord. They perform many functions, including biochemical control of endothelial cells that form the blood–brain barrier, provision of nutrients to the nervous tissue, maintenance of extracellular ion balance, regulation of cerebral blood flow, and a role in the repair and scarring process of the brain and spinal cord following infection and traumatic injuries. The proportion of astrocytes in the brain is not well defined; depending on the counting technique used, studies have found that the astrocyte proportion varies by region and ranges from 20% to around 40% of all glia. Another study reports that astrocytes are the most numerous cell type in the brain. Astrocytes are the major source of cholesterol in the central nervous system. Apolipoprotein E transports cholesterol from astrocytes to neurons and other glial cells, regulating cell signaling in the brain. Astrocytes in humans are more than twenty times larger than in rodent brains, and make contact with more than ten times the number of synapses.

Wallerian degeneration is an active process of degeneration that results when a nerve fiber is cut or crushed and the part of the axon distal to the injury degenerates. A related process of dying back or retrograde degeneration known as 'Wallerian-like degeneration' occurs in many neurodegenerative diseases, especially those where axonal transport is impaired such as ALS and Alzheimer's disease. Primary culture studies suggest that a failure to deliver sufficient quantities of the essential axonal protein NMNAT2 is a key initiating event.

Astrogliosis is an abnormal increase in the number of astrocytes due to the destruction of nearby neurons from central nervous system (CNS) trauma, infection, ischemia, stroke, autoimmune responses or neurodegenerative disease. In healthy neural tissue, astrocytes play critical roles in energy provision, regulation of blood flow, homeostasis of extracellular fluid, homeostasis of ions and transmitters, regulation of synapse function and synaptic remodeling. Astrogliosis changes the molecular expression and morphology of astrocytes, in response to infection for example, in severe cases causing glial scar formation that may inhibit axon regeneration.

Microglia are a type of neuroglia located throughout the brain and spinal cord. Microglia account for about 10-15% of cells found within the brain. As the resident macrophage cells, they act as the first and main form of active immune defense in the central nervous system (CNS). Microglia originate in the yolk sac under a tightly regulated molecular process. These cells are distributed in large non-overlapping regions throughout the CNS. Microglia are key cells in overall brain maintenance—they are constantly scavenging the CNS for plaques, damaged or unnecessary neurons and synapses, and infectious agents. Since these processes must be efficient to prevent potentially fatal damage, microglia are extremely sensitive to even small pathological changes in the CNS. This sensitivity is achieved in part by the presence of unique potassium channels that respond to even small changes in extracellular potassium. Recent evidence shows that microglia are also key players in the sustainment of normal brain functions under healthy conditions. Microglia also constantly monitor neuronal functions through direct somatic contacts and exert neuroprotective effects when needed.
Oligodendrocyte progenitor cells (OPCs), also known as oligodendrocyte precursor cells, NG2-glia, O2A cells, or polydendrocytes, are a subtype of glia in the central nervous system named for their essential role as precursors to oligodendrocytes. They are typically identified in the human by co-expression of PDGFRA and CSPG4.
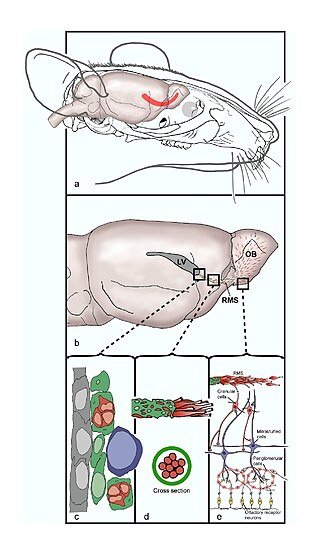
The rostral migratory stream (RMS) is a specialized migratory route found in the brain of some animals along which neuronal precursors that originated in the subventricular zone (SVZ) of the brain migrate to reach the main olfactory bulb (OB). The importance of the RMS lies in its ability to refine and even change an animal's sensitivity to smells, which explains its importance and larger size in the rodent brain as compared to the human brain, as our olfactory sense is not as developed. This pathway has been studied in the rodent, rabbit, and both the squirrel monkey and rhesus monkey. When the neurons reach the OB they differentiate into GABAergic interneurons as they are integrated into either the granule cell layer or periglomerular layer.
HIV-associated neurocognitive disorders (HAND) are neurological disorders associated with HIV infection and AIDS. It is a syndrome of progressive deterioration of memory, cognition, behavior, and motor function in HIV-infected individuals during the late stages of the disease, when immunodeficiency is severe. HAND may include neurological disorders of various severity. HIV-associated neurocognitive disorders are associated with a metabolic encephalopathy induced by HIV infection and fueled by immune activation of macrophages and microglia. These cells are actively infected with HIV and secrete neurotoxins of both host and viral origin. The essential features of HIV-associated dementia (HAD) are disabling cognitive impairment accompanied by motor dysfunction, speech problems and behavioral change. Cognitive impairment is characterised by mental slowness, trouble with memory and poor concentration. Motor symptoms include a loss of fine motor control leading to clumsiness, poor balance and tremors. Behavioral changes may include apathy, lethargy and diminished emotional responses and spontaneity. Histopathologically, it is identified by the infiltration of monocytes and macrophages into the central nervous system (CNS), gliosis, pallor of myelin sheaths, abnormalities of dendritic processes and neuronal loss.

The neuroimmune system is a system of structures and processes involving the biochemical and electrophysiological interactions between the nervous system and immune system which protect neurons from pathogens. It serves to protect neurons against disease by maintaining selectively permeable barriers, mediating neuroinflammation and wound healing in damaged neurons, and mobilizing host defenses against pathogens.
Neural stem cells (NSCs) are self-renewing, multipotent cells that firstly generate the radial glial progenitor cells that generate the neurons and glia of the nervous system of all animals during embryonic development. Some neural progenitor stem cells persist in highly restricted regions in the adult vertebrate brain and continue to produce neurons throughout life. Differences in the size of the central nervous system are among the most important distinctions between the species and thus mutations in the genes that regulate the size of the neural stem cell compartment are among the most important drivers of vertebrate evolution.

Interleukin 19 (IL-19) is an immunosuppressive protein that belongs to the IL-10 cytokine subfamily.

A gemistocyte is a swollen, reactive astrocyte.
Neuroregeneration involves the regrowth or repair of nervous tissues, cells or cell products. Neuroregenerative mechanisms may include generation of new neurons, glia, axons, myelin, or synapses. Neuroregeneration differs between the peripheral nervous system (PNS) and the central nervous system (CNS) by the functional mechanisms involved, especially in the extent and speed of repair. When an axon is damaged, the distal segment undergoes Wallerian degeneration, losing its myelin sheath. The proximal segment can either die by apoptosis or undergo the chromatolytic reaction, which is an attempt at repair. In the CNS, synaptic stripping occurs as glial foot processes invade the dead synapse.

A glial scar formation (gliosis) is a reactive cellular process involving astrogliosis that occurs after injury to the central nervous system. As with scarring in other organs and tissues, the glial scar is the body's mechanism to protect and begin the healing process in the nervous system.
Protective autoimmunity is a condition in which cells of the adaptive immune system contribute to maintenance of the functional integrity of a tissue, or facilitate its repair following an insult. The term ‘protective autoimmunity’ was coined by Prof. Michal Schwartz of the Weizmann Institute of Science (Israel), whose pioneering studies were the first to demonstrate that autoimmune T lymphocytes can have a beneficial role in repair, following an injury to the central nervous system (CNS). Most of the studies on the phenomenon of protective autoimmunity were conducted in experimental settings of various CNS pathologies and thus reside within the scientific discipline of neuroimmunology.
Gliogenesis is the generation of non-neuronal glia populations derived from multipotent neural stem cells.
Endogenous regeneration in the brain is the ability of cells to engage in the repair and regeneration process. While the brain has a limited capacity for regeneration, endogenous neural stem cells, as well as numerous pro-regenerative molecules, can participate in replacing and repairing damaged or diseased neurons and glial cells. Another benefit that can be achieved by using endogenous regeneration could be avoiding an immune response from the host.
Neuroinflammation is inflammation of the nervous tissue. It may be initiated in response to a variety of cues, including infection, traumatic brain injury, toxic metabolites, or autoimmunity. In the central nervous system (CNS), including the brain and spinal cord, microglia are the resident innate immune cells that are activated in response to these cues. The CNS is typically an immunologically privileged site because peripheral immune cells are generally blocked by the blood–brain barrier (BBB), a specialized structure composed of astrocytes and endothelial cells. However, circulating peripheral immune cells may surpass a compromised BBB and encounter neurons and glial cells expressing major histocompatibility complex molecules, perpetuating the immune response. Although the response is initiated to protect the central nervous system from the infectious agent, the effect may be toxic and widespread inflammation as well as further migration of leukocytes through the blood–brain barrier may occur.
Microglia are the primary immune cells of the central nervous system, similar to peripheral macrophages. They respond to pathogens and injury by changing morphology and migrating to the site of infection/injury, where they destroy pathogens and remove damaged cells.