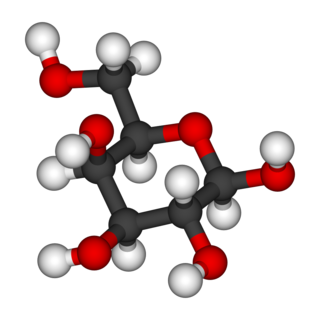
Galactose, sometimes abbreviated Gal, is a monosaccharide sugar that is about as sweet as glucose, and about 65% as sweet as sucrose. It is an aldohexose and a C-4 epimer of glucose. A galactose molecule linked with a glucose molecule forms a lactose molecule.
A glycosidic bond or glycosidic linkage is a type of ether bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate.
Glycosylation is the reaction in which a carbohydrate, i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule in order to form a glycoconjugate. In biology, glycosylation usually refers to an enzyme-catalysed reaction, whereas glycation may refer to a non-enzymatic reaction.
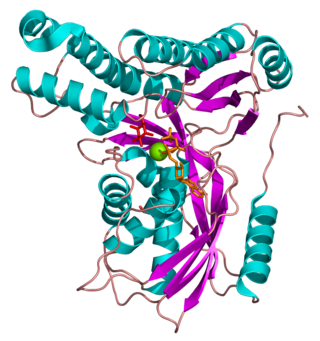
Galactokinase is an enzyme (phosphotransferase) that facilitates the phosphorylation of α-D-galactose to galactose 1-phosphate at the expense of one molecule of ATP. Galactokinase catalyzes the second step of the Leloir pathway, a metabolic pathway found in most organisms for the catabolism of α-D-galactose to glucose 1-phosphate. First isolated from mammalian liver, galactokinase has been studied extensively in yeast, archaea, plants, and humans.
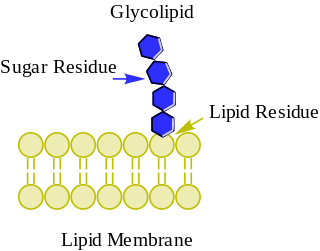
Glycolipids are lipids with a carbohydrate attached by a glycosidic (covalent) bond. Their role is to maintain the stability of the cell membrane and to facilitate cellular recognition, which is crucial to the immune response and in the connections that allow cells to connect to one another to form tissues. Glycolipids are found on the surface of all eukaryotic cell membranes, where they extend from the phospholipid bilayer into the extracellular environment.
An Endoglycosidase is an enzyme that releases oligosaccharides from glycoproteins or glycolipids. It may also cleave polysaccharide chains between residues that are not the terminal residue, although releasing oligosaccharides from conjugated protein and lipid molecules is more common.

Sialyltransferases are enzymes that transfer sialic acid to nascent oligosaccharide. Each sialyltransferase is specific for a particular sugar substrate. Sialyltransferases add sialic acid to the terminal portions of the sialylated glycolipids (gangliosides) or to the N- or O-linked sugar chains of glycoproteins.

The calicheamicins are a class of enediyne antitumor antibiotics derived from the bacterium Micromonospora echinospora, with calicheamicin γ1 being the most notable. It was isolated originally in the mid-1980s from the chalky soil, or "caliche pits", located in Kerrville, Texas. The sample was collected by a scientist working for Lederle Labs. It is extremely toxic to all cells and, in 2000, a CD33 antigen-targeted immunoconjugate N-acetyl dimethyl hydrazide calicheamicin was developed and marketed as targeted therapy against the non-solid tumor cancer acute myeloid leukemia (AML). A second calicheamicin-linked monoclonal antibody, inotuzumab ozogamicin an anti-CD22-directed antibody-drug conjugate, was approved by the U.S. Food and Drug Administration on August 17, 2017, for use in the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Calicheamicin γ1 and the related enediyne esperamicin are the two of the most potent antitumor agents known.

UTP—glucose-1-phosphate uridylyltransferase also known as glucose-1-phosphate uridylyltransferase is an enzyme involved in carbohydrate metabolism. It synthesizes UDP-glucose from glucose-1-phosphate and UTP; i.e.,

Galactosyltransferase is a type of glycosyltransferase which catalyzes the transfer of galactose. An example is B-N-acetylglucosaminyl-glycopeptide b-1,4-galactosyltransferase.

The enzyme UDP-glucose 4-epimerase, also known as UDP-galactose 4-epimerase or GALE, is a homodimeric epimerase found in bacterial, fungal, plant, and mammalian cells. This enzyme performs the final step in the Leloir pathway of galactose metabolism, catalyzing the reversible conversion of UDP-galactose to UDP-glucose. GALE tightly binds nicotinamide adenine dinucleotide (NAD+), a co-factor required for catalytic activity.
Nucleotide sugars are the activated forms of monosaccharides. Nucleotide sugars act as glycosyl donors in glycosylation reactions. Those reactions are catalyzed by a group of enzymes called glycosyltransferases.
In enzymology, an UDP-glucose—hexose-1-phosphate uridylyltransferase is an enzyme that catalyzes the chemical reaction
The gal operon is a prokaryotic operon, which encodes enzymes necessary for galactose metabolism. Repression of gene expression for this operon works via binding of repressor molecules to two operators. These repressors dimerize, creating a loop in the DNA. The loop as well as hindrance from the external operator prevent RNA polymerase from binding to the promoter, and thus prevent transcription. Additionally, since the metabolism of galactose in the cell is involved in both anabolic and catabolic pathways, a novel regulatory system using two promoters for differential repression has been identified and characterized within the context of the gal operon.
Histo-blood group ABO system transferase is an enzyme with glycosyltransferase activity, which is encoded by the ABO gene in humans. It is ubiquitously expressed in many tissues and cell types. ABO determines the ABO blood group of an individual by modifying the oligosaccharides on cell surface glycoproteins. Variations in the sequence of the protein between individuals determine the type of modification and the blood group. The ABO gene also contains one of 27 SNPs associated with increased risk of coronary artery disease.
Glycorandomization, is a drug discovery and drug development technology platform to enable the rapid diversification of bioactive small molecules, drug leads and/or approved drugs through the attachment of sugars. Initially developed as a facile method to manipulate carbohydrate substitutions of naturally occurring glycosides to afford the corresponding differentially glycosylated natural product libraries, glycorandomization applications have expanded to include both small molecules and even macromolecules (proteins). Also referred to as 'glycodiversification', glycorandomization has led to the discovery of new glycoside analogs which display improvements in potency, selectivity and/or ADMET as compared to the parent molecule.
O-linked glycosylation is the attachment of a sugar molecule to the oxygen atom of serine (Ser) or threonine (Thr) residues in a protein. O-glycosylation is a post-translational modification that occurs after the protein has been synthesised. In eukaryotes, it occurs in the endoplasmic reticulum, Golgi apparatus and occasionally in the cytoplasm; in prokaryotes, it occurs in the cytoplasm. Several different sugars can be added to the serine or threonine, and they affect the protein in different ways by changing protein stability and regulating protein activity. O-glycans, which are the sugars added to the serine or threonine, have numerous functions throughout the body, including trafficking of cells in the immune system, allowing recognition of foreign material, controlling cell metabolism and providing cartilage and tendon flexibility. Because of the many functions they have, changes in O-glycosylation are important in many diseases including cancer, diabetes and Alzheimer's. O-glycosylation occurs in all domains of life, including eukaryotes, archaea and a number of pathogenic bacteria including Burkholderia cenocepacia, Neisseria gonorrhoeae and Acinetobacter baumannii.
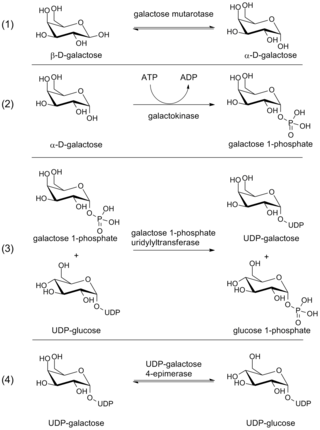
The Leloir pathway is a metabolic pathway for the catabolism of D-galactose. It is named after Luis Federico Leloir, who first described it.

Protein O-GlcNAc transferase also known as OGT or O-linked N-acetylglucosaminyltransferase is an enzyme that in humans is encoded by the OGT gene. OGT catalyzes the addition of the O-GlcNAc post-translational modification to proteins.
N-glycosyltransferase is an enzyme in prokaryotes which transfers individual hexoses onto asparagine sidechains in substrate proteins, using a nucleotide-bound intermediary, within the cytoplasm. They are distinct from regular N-glycosylating enzymes, which are oligosaccharyltransferases that transfer pre-assembled oligosaccharides. Both enzyme families however target a shared amino acid sequence asparagine—-any amino acid except proline—serine or threonine (N–x–S/T), with some variations.













